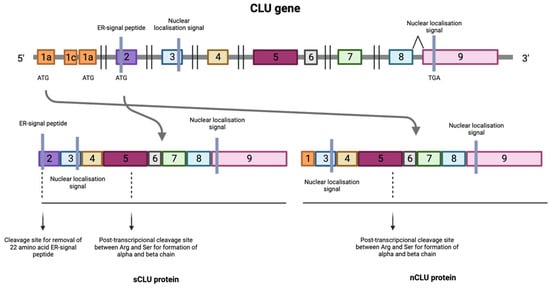
The regulation of CLU depends not only on the form of the protein, but also on the cell type. In addition to being found in Sertoli cells [
21], CLU can be found in a wide variety of cells, such as motor neurons [
22], dermal fibroblasts [
23] and epithelial cells [
24]. CLU expression in these varies considerably and this is thought to be due to both tissue type and different regulatory pathways acting in physiological or pathological situations [
2,
25]. Various factors, such as TGF-β, NGF (Nerve Growth Factor), EGF (epidermal growth factor), cytokines such as TNF-α and IL-1, IL-2, and IL-6, are involved in the regulation of CLU synthesis [
2]. The Wnt signaling pathway and transcription factors such as TCF1, Jak/STAT1 (Signal Transducers and Activators of Transcription 1) and IGF1 (Insulin-Like Growth Factor 1) also play a role in CLU expression [
26,
27]. In addition, ATM (Ataxia Telangiectasia-Mutated) has been found to act as a sensor of DNA damage, being necessary for the induction of the IGF1-sCLU axis after ionizing radiation [
28]. On the other hand, Ha-Ras and c-myc oncogenes can downregulate CLU expression [
10]. CLU regulation is mediated by several elements present in the CLU promoter region, such as AP-1 (Activator Protein-1), AP-2 (Activator Protein-2), SP-1 (Stimulatory Element-1), CLE (Clusterin Element), heat shock element (HSE)-like sequences, TCF (T-Cell Factor), NF-κB (Nuclear Factor Kappa-Light-Chain-Enhancer of Activated B Cells) and STAT1 binding sites [
29,
30]. CLU can also be epigenetically regulated by DNA methylation and histone acetylation due to the presence of CpG island-rich domains [
31,
32].
2. Colorectal Cancer
Colorectal cancer (CRC) is the third most frequently diagnosed cancer (10%) in both sexes, behind only breast cancer and lung cancer [
51]. It ranks second in terms of overall mortality, with a 65% survival rate [
52,
53]. Unfortunately, approximately 25% of patients present late for consultations, leading to diagnosis at advanced or metastatic stages and, therefore, to delayed treatment [
54,
55]. In 2019 alone, 60% of newly diagnosed cases were advanced disease, of which 22% had distant metastases [
56].
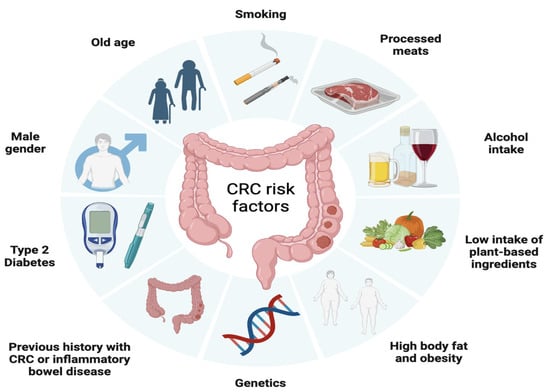
Similar to many other types of cancer, CRC risk is influenced by a range of health behaviors and lifestyle factors such as moderate to heavy alcohol consumption, smoking, a diet high in fat and low in vegetables, obesity, and sedentary lifestyle and non-modifiable factors such as age, ethnicity, and genetic predisposition [
57]. In fact, it is estimated that approximately 15% to 30% of CRC cases have a hereditary component in first- and second-degree relatives [
58], with a higher risk observed in individuals who have first- and second-degree relatives affected by CRC. In addition, inflammatory bowel diseases, such as Crohn’s disease and ulcerative colitis, increase the risk of developing CRC, especially when the inflammation is chronic and long-lasting [
59] (
Figure 2).
Figure 2. Factors associated with the development of colorectal cancer including modifiable factors (moderate to heavy alcohol consumption, smoking, a diet high in fat and low in vegetables, obesity, and sedentary lifestyle), non-modifiable factors (age, ethnicity, inflammatory bowel disease) and hereditary component.
The progression of colorectal cancer follows a multi-step process, starting from normal epithelial cells and advancing through distinct stages, from the formation of a premalignant lesion, commonly known as an adenoma, into a malignant lesion referred to as carcinoma. Carcinomas are characterized by invasive growth into surrounding tissues and eventually, if left unchecked, can spread systemically.
Advancements in understanding the molecular genetics and epigenetics of colorectal cancer has led to the identification of well-established alterations related to CRC such as chromosomal instability, microsatellite instability (MSI-H) and the CpG island methylator (CGI) phenotype stand out [
60].
Chromosomal instability, present in 84% of cases, is related to the activation of oncogenes such as PIK3CA, K-RAS, APC, SMAD4 and TP53 [
61]. Mutations in the APC oncogene occur in 80% of CRC cases [
62] and lead to WNT signaling pathway activation, which promotes cells proliferation and differentiation [
63]. While transformation from adenoma to carcinoma is usually caused by mutations in TP53, K-RAS and DCC 18q genes [
64], progression to metastasis is normally associated with the accumulation of genetic changes in APC-KRAS-TP53 (Adenomatous Polyposis Coli–Kirsten rat sarcoma viral oncogene- the tumor suppressor p53), according to the Vogelstein model [
65]. In addition, approximately 10% of CRC cases are caused by serrated neoplasia, characterized by mutations in the BRAF and K-RAS genes, which activates the MAPKinase signaling pathway, which has a main role in the regulation of gene expression, cellular growth, and survival [
62,
66,
67].
Microsatellite instability (MSI-H) is present in 15% to 20% of CRC cases and is due to hypermethylation of the hMSH2 and hMLH1 promoters [
68], as well as to mutations in mismatch repair (MMR) genes. On the other hand, the CpG-rich island (CGI) methylating phenotype involves methylation of the 5’ ends of half of all genes with short, CpG dinucleotide-rich sequences [
69].
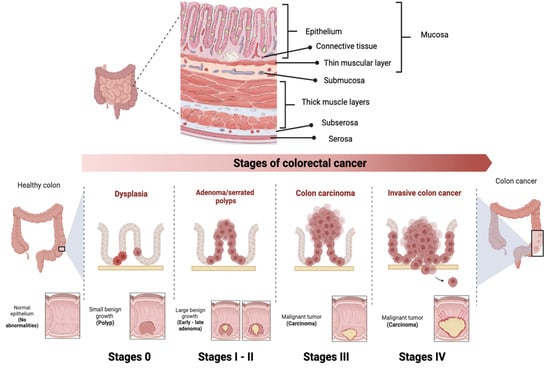
CRC is classified into different stages depending on the extent of the tumor and the presence of metastases (
Figure 3) [
70]. Stage 0 (carcinoma in situ) implies that the cancer cells are found only in the innermost layer of the lining of the colon or rectum, without invading nearby tissues or spreading to lymph nodes or other parts of the body. In stage I, cancer has grown through the innermost layer of the lining, but has not spread to lymph nodes or distant organs. In stage II, cancer has grown through the lining of the colon or rectum, but has not spread to lymph nodes or distant organs. In stage III, the cancer has invaded nearby lymph nodes, but has not reached distant organs. In stage IV, the cancer has spread to distant organs. Prognosis and treatment vary at each stage, and may include surgery, radiation therapy, chemotherapy, and targeted therapy or immunotherapy [
71].
Figure 3. Stages of colorectal cancer depending on the extent of the tumor and the presence of metastases. The TNM staging system is used to evaluate cancer and determine its stage. It focuses on Tumor (T), describing the depth of growth of the primary tumor into the intestinal lining, with categories ranging from T0 (no cancer) to T4b (invasion of other organs or structures). The results of T, along with the assessment of Lymph Nodes (N) and Metastases (M), are combined to assign a stage to the cancer, ranging from 0 to IV.
Since the 5-year survival rate is 91% for stage I, decreasing to 72% for locally advanced stage disease and dropping further to 14% for stage IV [
56], there is a current need for the improvement of CRC prevention and screening programs that allow for early detection. Nowadays, colonoscopy is the first option for the patient at is at higher risk of CRC due to genetic syndromes, personal or family history of colorectal cancer or the presence of precancerous polyps. Despite being a relatively expensive invasive procedure, with limitations due to the intestinal preparation required and the complications of the procedure itself [
72], colonoscopy represent the most decisive test for detecting CRC because it allows the entire colon to be analyzed, biopsies of suspicious lesions to be obtained and the polyp to be removed in the same session. with a 5% miss rate.
If the patient is not at high risk, the first option is the test based on the detection of blood in stool. Despite its low sensitivity, this test was the first one used for population screening because it is the most economical and the least invasive so far available, reason why it is currently being replaced by methods such as the fecal immunohistochemical test [
72]. This test was firstly approved in 2014 by the US Food and Drug Administration (FDA) and recommended for screening tests in asymptomatic patients aged 50–85 years [
73].
Conventional treatments used to treat CRC include surgery, chemotherapy, and radiotherapy, alone or in combination, depending on the location [
71]:
Total excision through surgery is the option used to treat localized CRC if the tumor location is easily accessible [
74]. Since complete elimination of all cancer cells is not always possible so approximately 66% of stage II and III CRC patients must undergo additional treatments, where adjuvant chemotherapy and/or radiotherapy are included, respectively [
75] and, in addition, 54% of patients often relapse even after undergoing adjuvant treatment [
76]. This evidences the need for alternative treatments and more effects to treat CRC patients [
74].
Neoadjuvant radiation therapy is another important clinical option available to treat CRC, especially for those at an intermediate or advanced stage for whom surgery is not feasible or chemotherapy cannot be tolerated well. However, the use of radiotherapy is quite limited as it has low sensitivity for CRC and high toxicity in surrounding healthy tissues [
77]. This neoadjuvant treatment can be given as a short course followed by surgery or as chemoradiotherapy with 5-fluorouracil or capecitabine, where possible [
78].
The first chemotherapeutic used against CRC was 5-fluorouracil (5-FU) [
79]. Its combination with leucovorin became the standard for metastatic colon and rectal cancer (mCRCC) allowing a median overall survival of 8 to 9 months. Years later, the approval of oxaliplatin and the demonstration that it induces cell death by immunogenic mechanisms, allowed the establishment of the current standard chemotherapy of 5-FU and oxilaplatin, commonly known as FOLFOX, which has demonstrated a has superior efficacy with a median survival of 18 to 20 months [
80,
81].
Recently, the Chinese Clinical Oncological Society has recommended the application of neoadjuvant chemoradiotherapy in patients with CRC, specifically with sigmoid colon cancer, and with a locally advanced stage (T4b) since the response of such treatment can be increased from 26.3% to 38.1%. However, whether MSI-H patients can benefit from this approach, remains as a controversial issue [
82]. Postoperative adjuvant chemotherapy is recommended for all stage III CRC [
83].
Monoclonal Antibodies
After 20 years of translational and clinical research, the epidermal growth factor receptor (EGFR) family and its intracellular signaling pathways constitute one of the foundations of molecular targeted therapy for CRC [
84]. EGFR serve as cell-surface protein receptors for the peptide ligands of the epidermal growth factor (EGF) family with enzymatic tyrosine kinase activity [
84]. Ligand binding induces receptor conformational change, activation, and subsequent phosphorylation of intracellular tyrosine residues, which leads to the activation of different intracellular pathways including the RAS-RAF-MEK-MAPK and PTEN-I3K-AKT-mTOR pathways, which play a main role the regulation of cell proliferation, survival, dissemination, and angiogenesis [
85]. Given the role of these processes in cancer development and tumor progression, targeting the EGFR has become a key strategy in the treatment of metastatic colorectal cancer (mCRC) [
86,
87].
For its part, the RAS family comprises four genes (
KRAS4A,
KRAS4B,
HRAS and
NRAS) which are among the most frequently altered oncogenes in human cancer, encode for four proteins with pivotal roles in cells signaling. KRAS4A (K-RAS), the predominant splice variant, and part of the RAS/MAPK pathway. Upon stimulation by upstream receptors such as EGFR, KRAS switches from inactive to active state, and promotes RAF recruitment to the cell membrane, leading to RAF dimerization and activation of downstream effector pathways.
KRAS mutations, which are present in approximately 40% of CRC cases, determine constitutive activation of RAS, and promote tumorigenesis as well as modulation of the tumor microenvironment by inducing immune escape and cancer progression [
88], reason why RAS mutations are generally associated with poor prognosis and low response to conventional CRC therapies [
89]. On the other hand, BRAF mutations, which are seen in 10% to 15% of CRC cases [
90], usually lead to constitutive activation of the mitogen-activated protein kinase (MAPK) signaling pathway, conferring high clinical aggressiveness, resistance to anti-EGFR monoclonal antibody therapy, and poor survival [
91].
The first successful step towards personalized cancer medicine has been the definition of different treatment options and sequences, which are based on tumor molecular stratification [
92]. Since the validation of
KRAS and
BRAF mutations as predictive biomarkers to anti-EGFR monoclonal antibodies in mCRC, regulatory agencies, such as the FDA and the European Medicines Agency (EMA), established the need to assess K-RAS and BRAF mutation status for patient stratification and management.
Bevacizumab (Avastin; Genentech, South San Francisco, CA, USA), a human antiendothelial growth factor receptor 2 (VEGF) monoclonal antibody, anti-VEGF-A, was the first antigenic drug to be successfully added to the therapeutic armamentarium for CRC. Given its very modest action as a single agent the FDA approves its use in combination with fluoropyrimidine [
93]. Nowadays, bevacizumab is widely used for the treatment of patients with mCRC in combination with oxaliplatin-based chemotherapy, due to evidence of improved patient survival [
94], either as first-line or second-line therapeutic options [
95,
96].
Otros medicamentos disponibles aprobados como tratamiento de segunda línea para el CCRm, Ziv-aflibercept (Zaltrap; Regeneron Pharmaceuticals, Tarrytown, NY, EE. UU.), una proteína de fusión recombinante totalmente humanizada que bloquea el VEGF-A con mayor afinidad de unión que Bevacizumad [97] y
Ramucirumab ( Cyramza; Eli Lilly, Indianápolis, IN, EE. UU.) un anticuerpo monoclonal (mAb) IgG1 completamente humanizado dirigido al dominio extracelular de VEGF, los cuales han demostrado eficacia para uso de segunda línea contra el CCR en combinación con leucovorina e ironotecán (FOLFIRI) o irinotecán solo.