Marine bacterial species contribute to a significant part of the oceanic population, which substantially produces biologically effectual moieties having various medical and industrial applications. The use of marine-derived bacterial pigments displays a snowballing effect, being natural, environmentally safe, and health beneficial compounds. Marine-derived bacterial pigments serve as valuable products in the food, pharmaceutical, textile, and cosmetic industries due to their beneficial attributes, including anticancer, antimicrobial, antioxidant, and cytotoxic activities. Biodegradability and higher environmental compatibility further strengthen the use of marine bio-pigments over artificially acquired colored molecules. Besides that, hazardous effects associated with the consumption of synthetic colors further substantiated the use of marine dyes as color additives in industries as well.
- natural colors
- bio-pigments
- quorum sensing
- marine bacteria
- biosynthesis
- biological activities
- industrial applications
- therapeutic insights
1. Introduction
2. Marine Bacterial Species as Sources of Bio-Pigments
|
Pigments |
Marine Bacterial Species |
References |
|---|---|---|
|
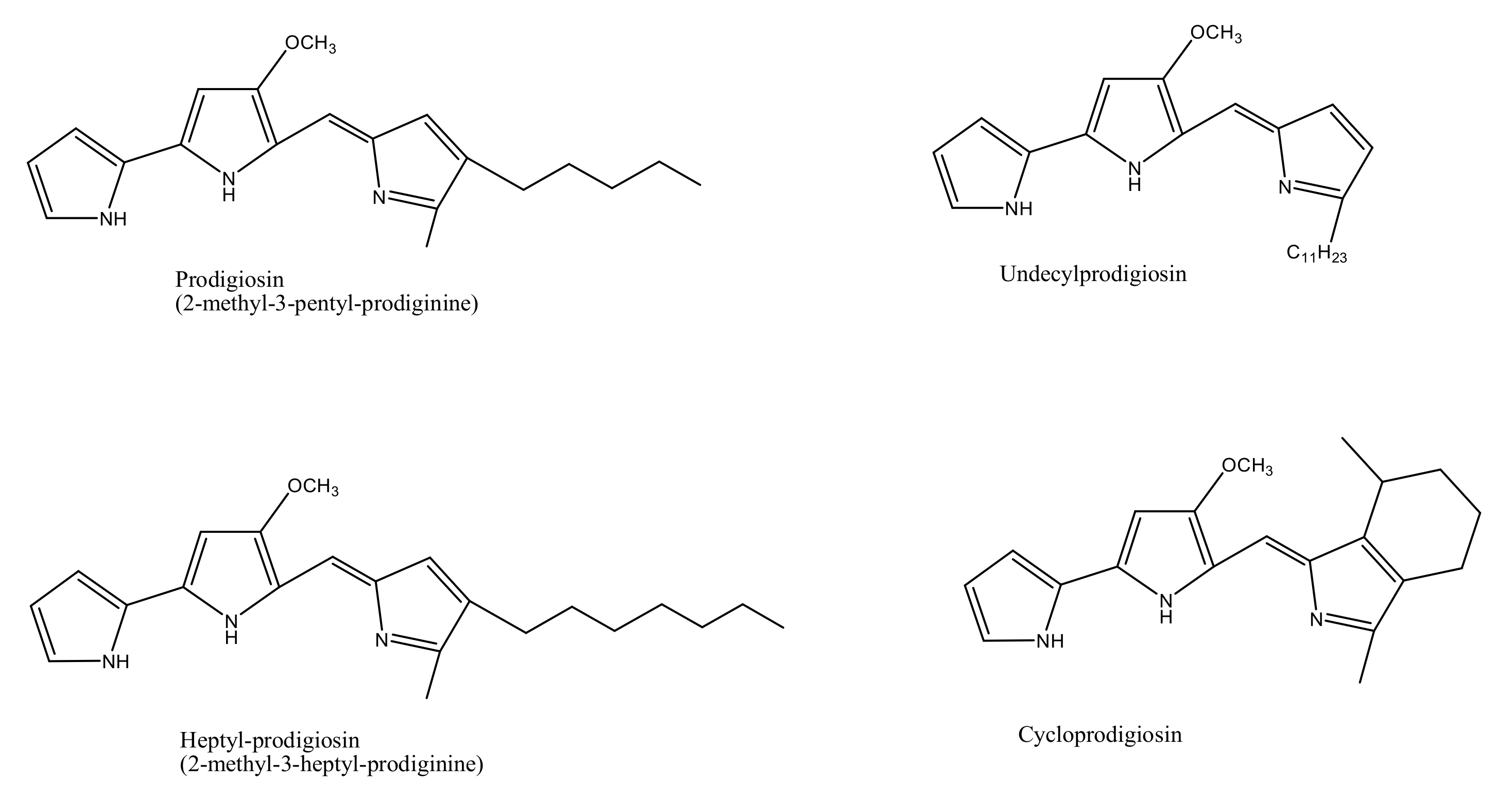
Prodigiosin |
Hahella chejuensis sp. Pseudoalteromonas rubra sp. Streptomyces sp. SCSIO 11594 Vibrio sp. (Strain MI-2) Serratia marcescens sp. IBRL USM 84 Zooshikella ganghwensis gen. nov., sp. nov. |
|
|
Undecylprodigiosin |
Streptomyces sp. UKMCC_PT15 Streptomyces sp.SCSIO 11594 Novel strain of Actinobacterium sp., Saccharopolyspora sp. |
|
|
Heptylprodigiosin |
Spartinivicinus ruber gen. nov., sp. nov |
[14] |
|
Cycloprodigiosin |
Pseudoalteromonas denitrificans sp. Pseudoalteromonas rubra sp. ATCC 29570 |
|
|
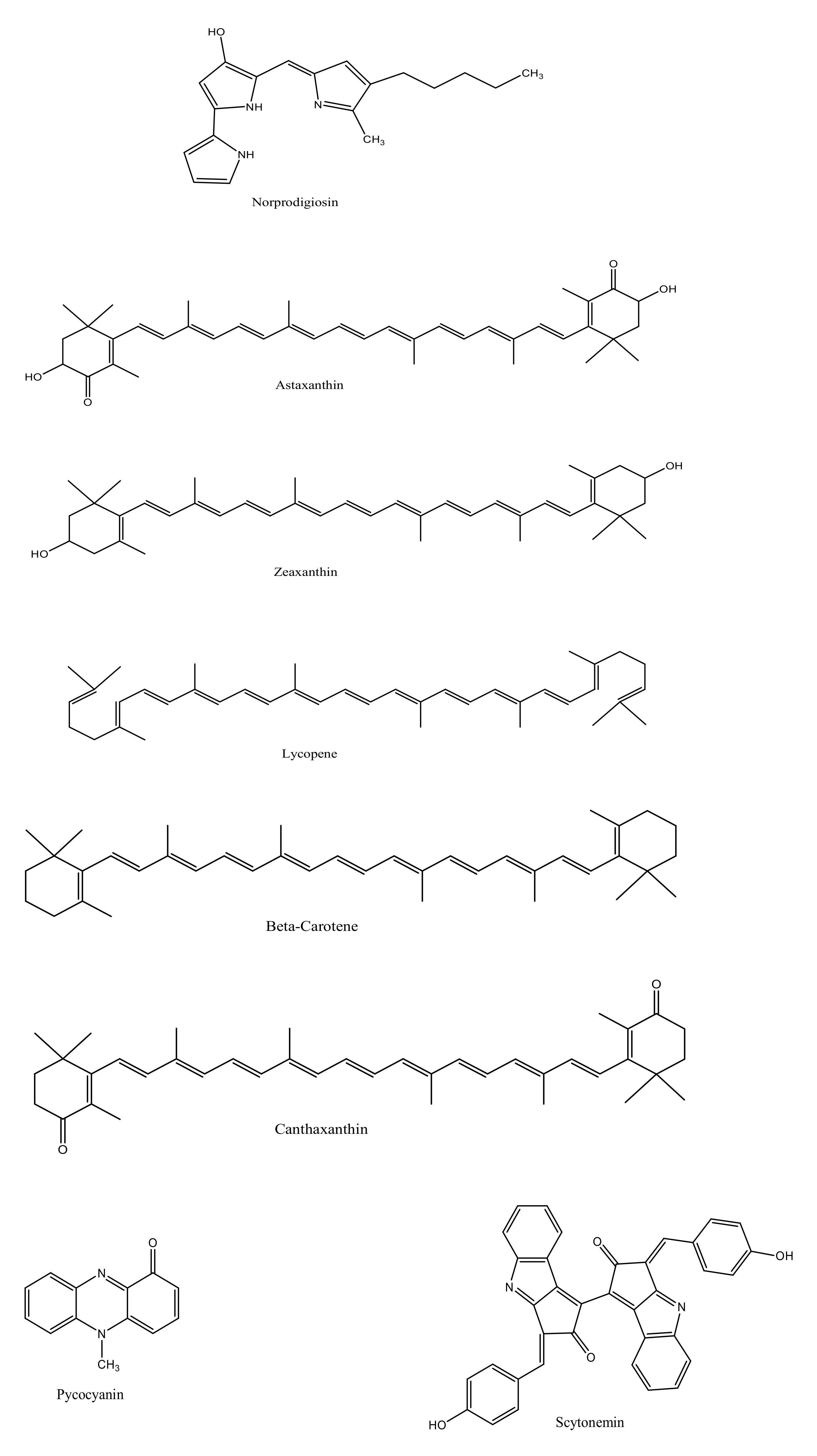
Norprodigiosin |
Serratia sp. WPRA3 |
[17] |
|
Astaxanthin |
Brevundimonas scallop sp. Zheng & Liu Corynebacterium glutamicum sp. Brevundimonas sp. strain N-5 Sphingomicrobium astaxanthinifaciens sp. nov Rhodovulum sulfidophilum sp. Pontibacter korlensis sp. AG6 Exiguobacterium sp. Altererythrobacter ishigakiensis sp. NBRC 107699 Rhodotorula sp. Paracoccus haeundaensis sp. |
|
|
Zeaxanthin |
Sphingomonas phyllosphaerae sp. KODA19-6 Mesoflavibacter aestuarii sp. nov. Aquibacter zeaxanthinifaciens gen. nov., sp. nov. Zeaxanthinibacter enoshimensis gen. nov., sp. nov Gramella planctonica sp. nov Mesoflavibacter zeaxanthinifaciens gen. nov., sp. nov Formosa sp. KMW Sphingomonas phyllosphaerae sp. FA2T Sphingomonas (Blastomonas) natatoria sp. DSM 3183T Muricauda lutaonensis sp. CC-HSB-11T |
|
|
Lycopene |
Blastochloris tepida sp. Salinicoccus roseus sp. |
|
|
Beta carotene |
Cyanobacterium Synechococcus sp. Micrococcus sp. Vibrio owensii sp. Flavicella marina gen. nov., sp. nov. Gordonia terrae sp.TWRH01 |
|
|
Canthaxanthin |
Brevibacterium sp. Gordonia sp. Dietzia sp. |
[39] |
|
Pyocyanin |
Pseudomonas aeruginosa sp. |
[43] |
|
Scytonemin |
Cyanobacterial sp. Nostoc punctiforme sp. ATCC |
|
|
Violacein |
Pseudoalteromonas luteoviolacea sp. Pseudoalteromonas ulvae sp. TC14 Pseudoalteromonas sp. 520P1 Microbulbifer sp. D250 Pseudoalteromonas amylolytica sp. nov Chromobacterium violaceum sp. Collimonas sp. |
|
|
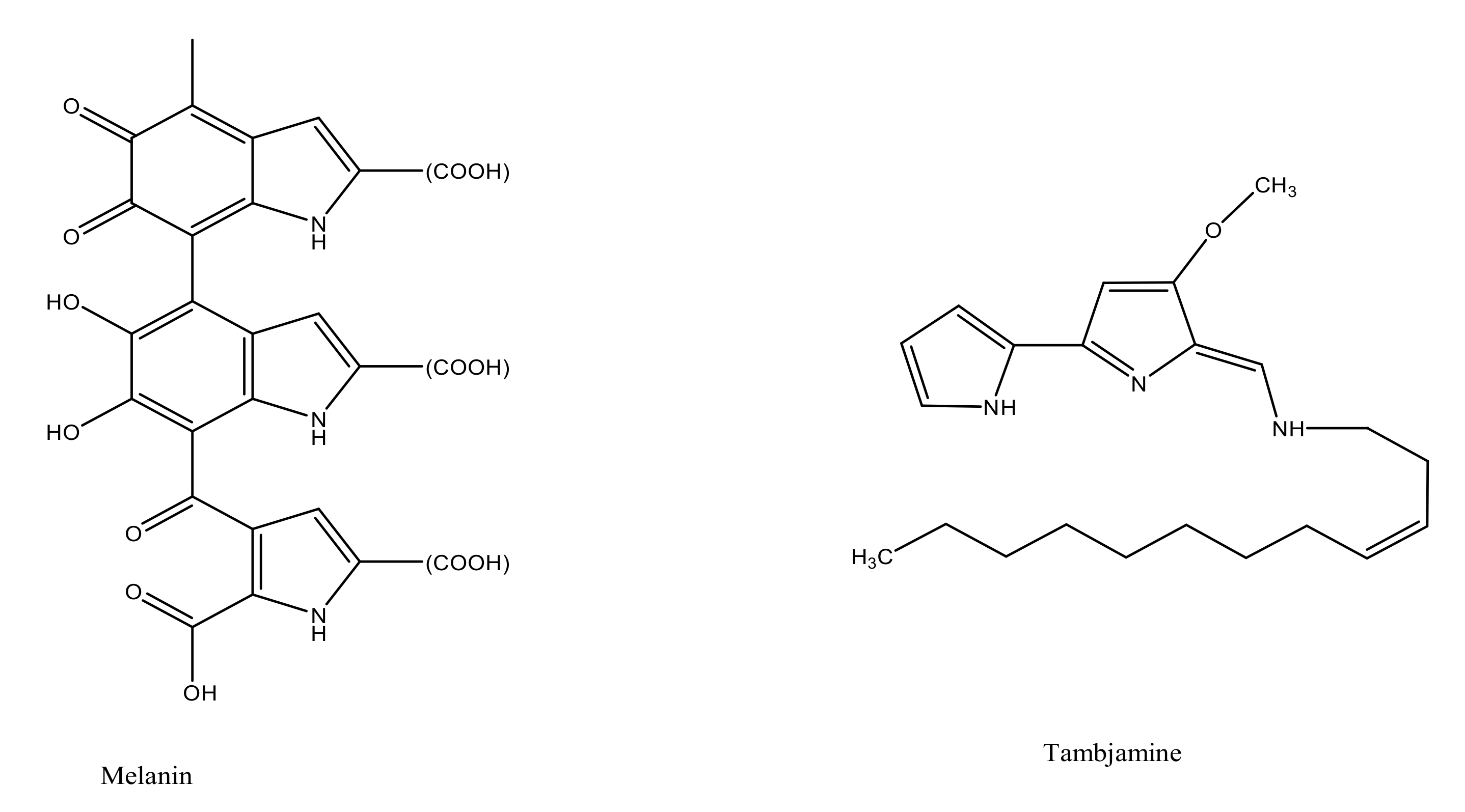
Melanin |
Streptomyces sp. Pseudomonas sp. Marinomonas mediterranea sp. MMB-1T Pseudomonas stutzeri sp. Bacillus sp. BTCZ31 Streptomyces sp. MVCS13 Providencia rettgeri sp. strain BTKKS1 Marinobacter alkaliphilus sp. Leclercia sp. Halomonas meridian sp. Nocardiopsis dassonvillei sp. strain JN1 Vibrio alginolyticus sp. strain BTKKS3 |
|
|
Tambjamines |
Pseudoalteromonas tunicata sp. Pseudoalteromonas citrea sp. |
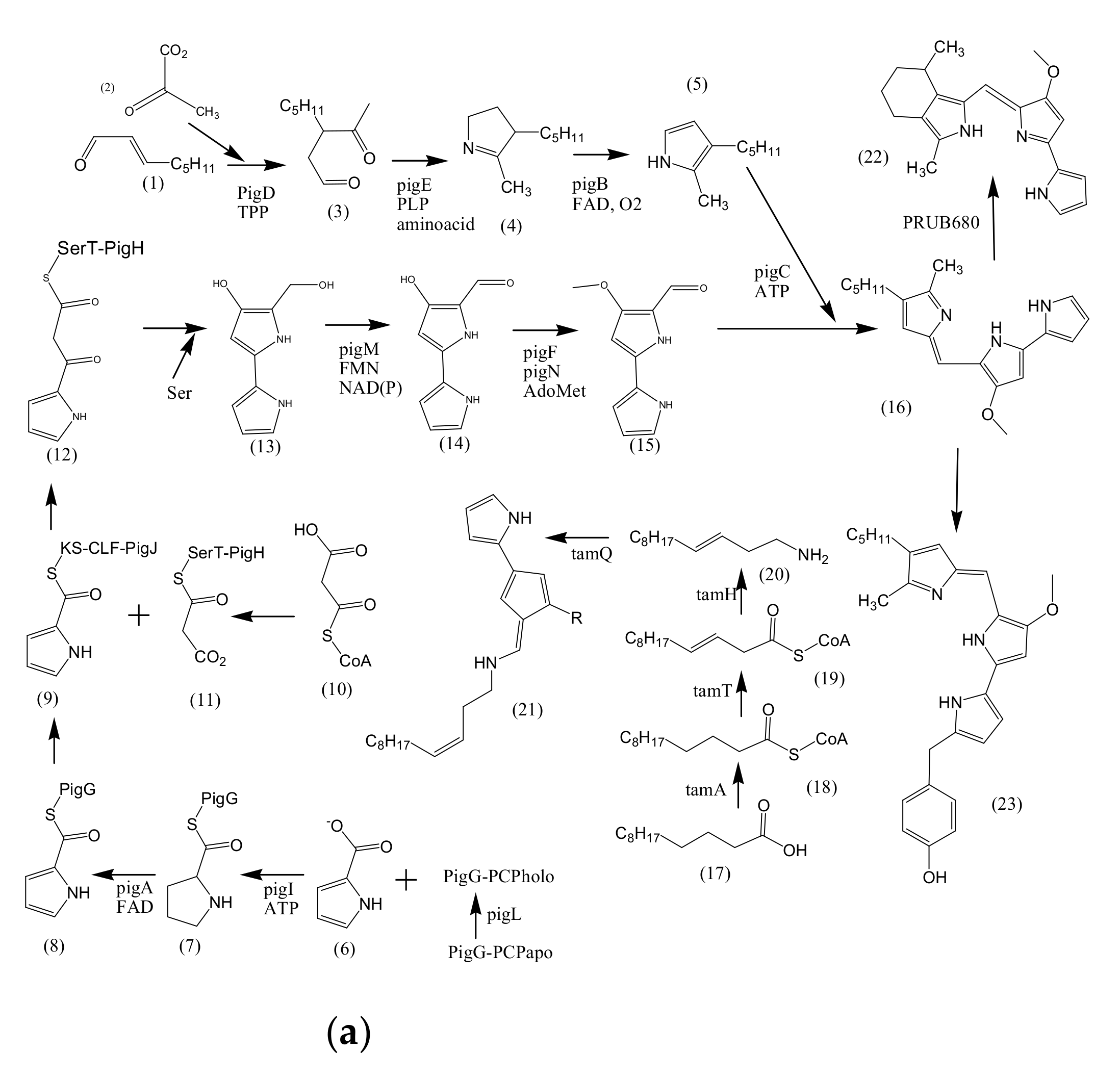
3. Biosynthesis of Bacterial Pigments
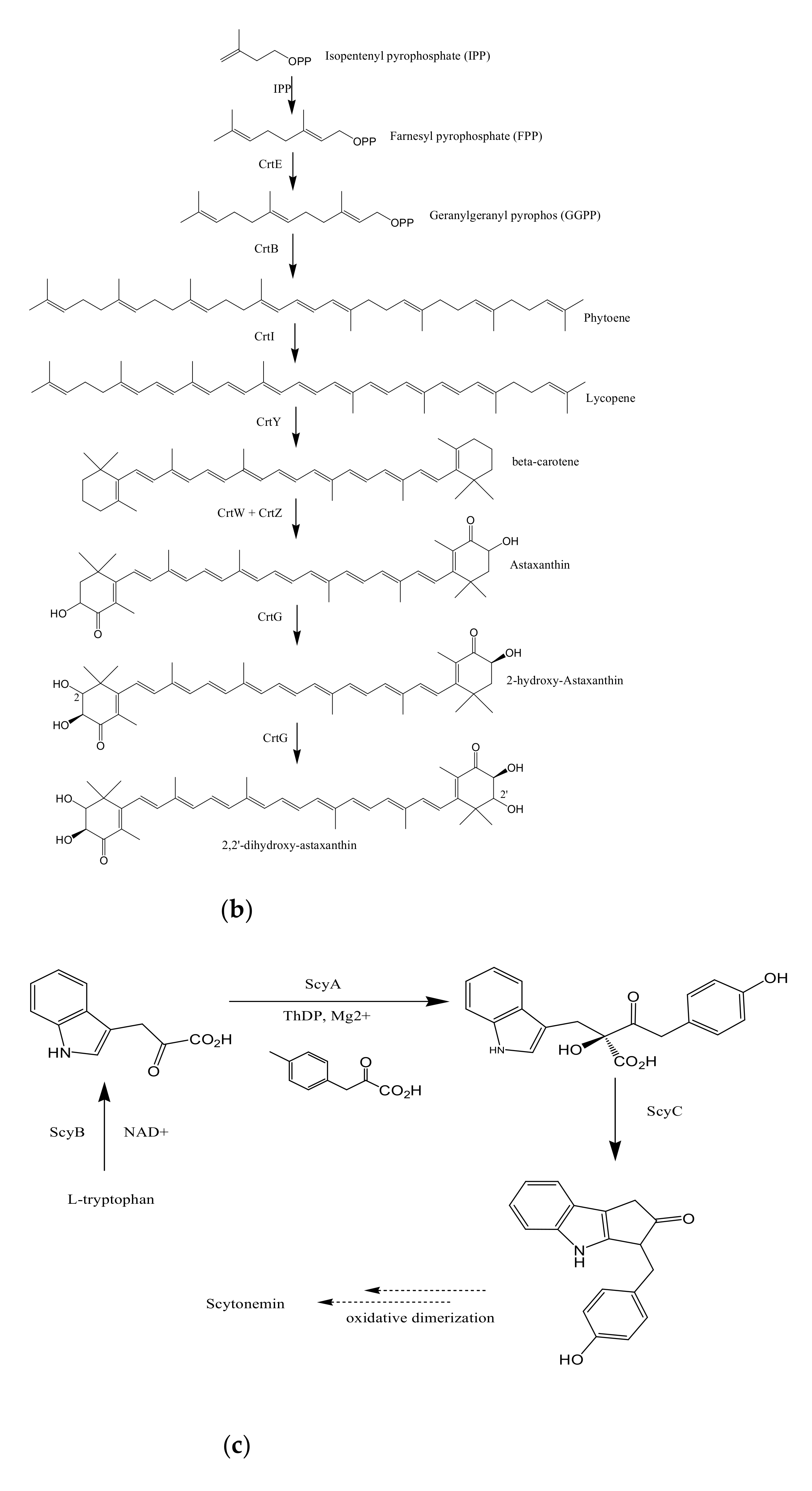
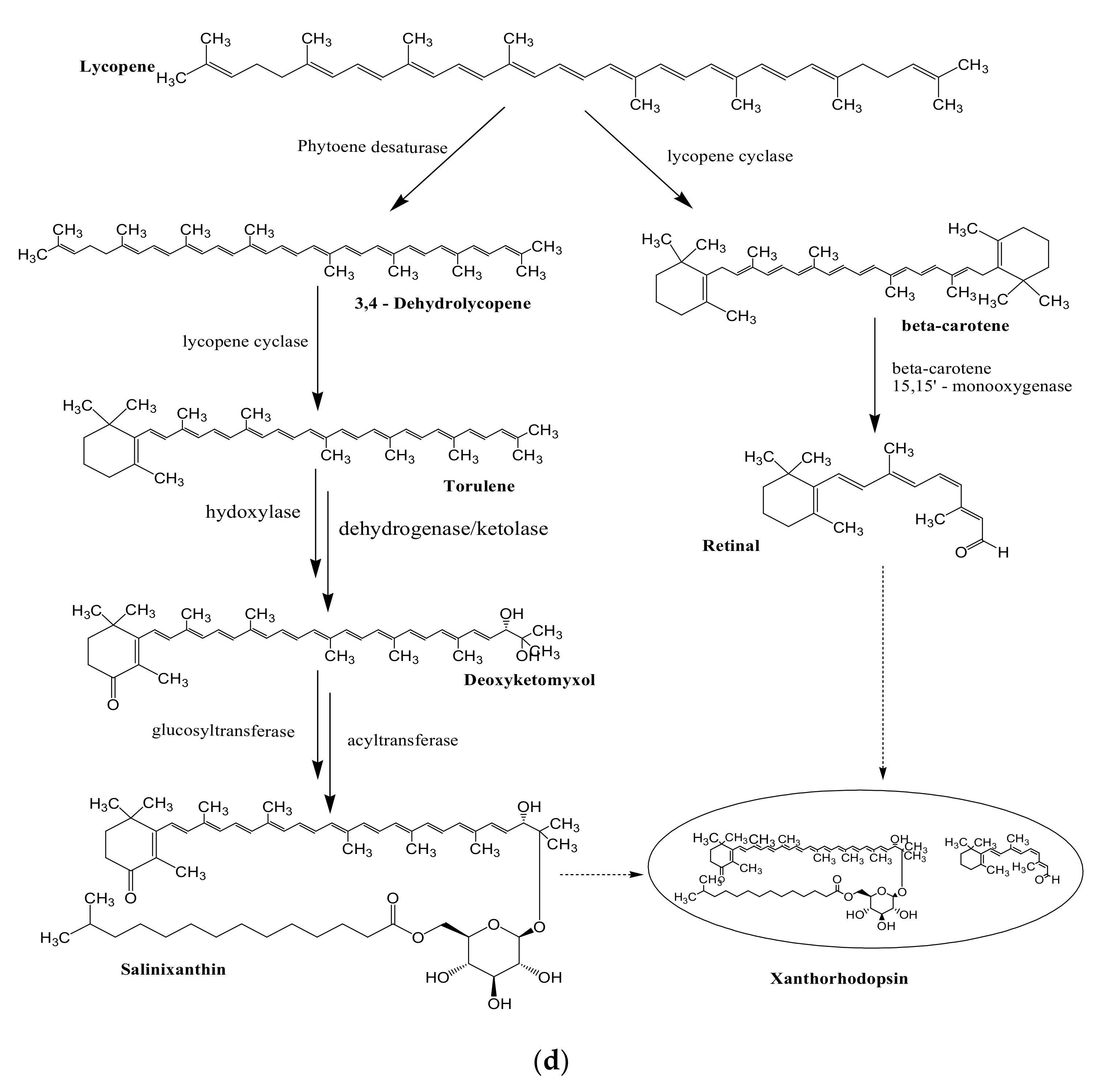
4. Industrial and Therapeutic Applications
4.1. Therapeutic Applications
4.1.1. Antibacterial Activity
4.1.2. Antifungal Activity
4.1.3. Anticancer Activity
4.1.4. Antioxidant Activity
4.1.5. Antiviral Activity
4.2. Industrial Applications
4.2.1. Bio-Pigments as Food Colorants
4.2.2. Bio-Pigments as Dyeing Agents
4.2.3. Use in Cosmetics
4.2.4. Antifouling Agent
4.2.5. Photosensitizers
This entry is adapted from the peer-reviewed paper 10.3390/microorganisms9010011
References
- Ramesh, C.; Vinithkumar, N.V.; Kirubagaran, R. Marine Pigmented Bacteria: A Prospective Source of Antibacterial Compounds. J. Nat. Sci. Biol. Med. 2019, 10, 104–113.
- Venil, C.K.; Zakaria, Z.A.; Ahmad, W.A. Bacterial Pigments and Their Applications. Process Biochem. 2013, 48, 1065–1079.
- Saviola, B. Pigments and Pathogenesis. J. Mycobact. Dis. 2014, 4, 5.
- Shindo, K.; Misawa, N. New and Rare Carotenoids Isolated from Marine Bacteria and Their Antioxidant Activities. Mar. Drugs 2014, 12, 1690–1698.
- Pierson, L.S.; Pierson, E.A. Metabolism and Function of Phenazines in Bacteria: Impact on the Behavior of Bacteria in the Environment and Biotechnological Process. Appl. Microbiol. Biotechnol. 2010, 86, 1659–1670.
- Kim, D.; Kim, J.F.; Yim, J.H.; Kwon, S.-K.; Lee, C.H.; Lee, H.K. Red to Red—The Marine Bacterium Hahella chejuensis and Its Product Prodigiosin for Mitigation of Harmful Algal Blooms. J. Microbiol. Biotechnol. 2008, 18, 1621–1629.
- Setiyono, E.; Adhiwibawa, M.A.S.; Indrawati, R.; Prihastyanti, M.N.U.; Shioi, Y.; Brotosudarmo, T.H.P. An Indonesian Marine Bacterium, Pseudoalteromonas rubra, Produces Antimicrobial Prodiginine Pigments. ACS Omega 2020, 5, 4626–4635.
- Song, Y.; Liu, G.; Li, J.; Huang, H.; Zhang, X.; Zhang, H.; Ju, J. Cytotoxic and Antibacterial Angucycline- and Prodigiosin-Analogues from the Deep-Sea Derived Streptomyces sp. SCSIO 11594. Mar. Drugs 2015, 13, 1304–1316.
- Morgan, S.; Thomas, M.J.; Walstrom, K.M.; Warrick, E.C.; Gasper, B.J. Characterization of Prodiginine Compounds Produced by a Vibrio species Isolated from Salt Flat Sediment along the Florida Gulf Coast. Fine Focus 2016, 3, 33–51.
- Ibrahim, D.; Nazari, T.F.; Kassim, J.; Lim, S.-H. Prodigiosin—An Antibacterial Red Pigment Produced by Serratia marcescens IBRL USM 84 Associated with a Marine Sponge Xestospongia testudinaria. J. Appl. Pharm. Sci. 2014, 4, 1–6.
- Yi, H.; Chang, Y.-H.; Oh, H.W.; Bae, K.S.; Chun, J. Zooshikella ganghwensis gen. nov., sp. nov., Isolated from Tidal Flat Sediments. Int. J. Syst. Evol. Microbiol. 2003, 53, 1013–1018.
- Abidin, Z.A.Z.; Ahmad, A.; Latip, J.; Usup, G. Marine Streptomyces sp. UKMCC_PT15 Producing Undecylprodigiosin with Algicidal Activity. J. Teknol. 2016, 78, 11-2.
- Bramhachari, P.V.; Mutyala, S.; Bhatnagar, I.; Pallela, R. Novel Insights on the Symbiotic Interactions of Marine Sponge-Associated Microorganisms: Marine Microbial Biotechnology Perspective. In Marine Sponges: Chemicobiological and Biomedical Applications, 1st ed.; Pallela, R., Ehrlich, H., Eds.; Springer: New Delhi, India, 2016; pp. 69–95.
- Huang, Z.; Dong, L.; Lai, Q.; Liu, J. Spartinivicinus ruber gen. nov., sp. nov., a Novel Marine Gamma proteobacterium Producing Heptylprodigiosin and Cycloheptylprodigiosin as Major Red Pigments. Front. Microbiol. 2020, 11, 11.
- Soliev, A.B.; Hosokawa, K.; Enomoto, K. Bioactive Pigments from Marine Bacteria: Applications and Physiological Roles. Evid. Based Complement. Altern. Med. 2011, 1–17.
- Xie, B.-B.; Shu, Y.-L.; Qin, Q.-L.; Rong, J.-C.; Zhang, X.-Y.; Chen, X.-L.; Zhou, B.-C.; Zhang, Y.-Z. Genome Sequence of the Cycloprodigiosin-Producing Bacterial Strain Pseudoalteromonas rubra ATCC 29570 T. J. Bacteriol. 2012, 194, 1637–1638.
- Jafarzade, M.; Yahya, N.A.; Shayesteh, F.; Usup, G.; Ahmad, A. Influence of Culture Conditions and Medium Composition on the Production of Antibacterial Compounds by Marine Serratia sp. WPRA3. J. Microbiol. 2013, 51, 373–379.
- Liu, H.; Zhang, C.; Zhang, X.; Tana, K.; Zhang, H.; Cheng, D.; Ye, T.; Li, S.; Ma, H.; Zheng, H. A Novel Carotenoids-Producing Marine Bacterium from Noble Scallop Chlamys nobilis and Antioxidant Activities of Its Carotenoid Compositions. Food Chem. 2020, 320, 126629.
- Henke, N.A.; Heider, S.A.E.; Peters-Wendisch, P.; Wendisch, V.F. Production of the Marine Carotenoid Astaxanthin by Metabolically Engineered Corynebacterium glutamicum. Mar. Drugs 2016, 14, 124.
- Asker, D. Isolation and Characterization of a Novel, Highly Selective Astaxanthin-Producing Marine Bacterium. J. Agric. Food Chem. 2017, 65, 9101–9109.
- Shahina, M.; Hameed, A.; Lin, S.-Y.; Hsu, Y.-H.; Liu, Y.-C.; Cheng, I.-C.; Lee, M.-R.; Lai, W.-A.; Lee, R.-J.; Young, C.-C. Sphingomicrobium astaxanthinifaciens sp. nov., an Astaxanthin-Producing Glycolipid-Rich Bacterium Isolated from Surface Seawater and Emended Description of the Genus Sphingomicrobium. Int. J. Syst. Evol. Microbiol. 2013, 63, 3415–3422.
- Mukoyama, D.; Takeyama, H.; Kondo, Y.; Matsunaga, T. Astaxanthin Formation in the Marine Photosynthetic Bacterium Rhodovulum sulfidophilum Expressing crtI, crtY, crtW and crtZ. FEMS Microbiol. Lett. 2006, 265, 69–75.
- Pachaiyappan, A.; Sadhasivam, G.; Kumar, M.; Muthuvel, A. Biomedical Potential of Astaxanthin from Novel Endophytic Pigment Producing Bacteria Pontibacter korlensis AG6. Waste Biomass Valoriz. 2020, 1–11.
- Balraj, J.; Pannerselvam, K.; Jayaraman, A. Isolation of Pigmented Marine Bacteria Exiguobacterium sp. from Peninsular Region of India and a Study on Biological Activity of Purified Pigment. Int. J. Sci. Techol. Res. 2014, 3, 375–384.
- Shi, X.-L.; Wu, Y.-H.; Cheng, H.; Zhang, X.-Q.; Wang, C.-S.; Xu, X.-W. Complete Genome Sequence of Astaxanthin-Producing Bacterium Altererythrobacter ishigakiensis. Mar. Genom. 2016, 30, 77–79.
- Zhao, Y.; Guo, L.; Xia, Y.; Zhuang, X.; Chu, W. Isolation, Identification of Carotenoid-Producing Rhodotorula sp. from Marine Environment and Optimization for Carotenoid Production. Mar. Drugs 2019, 17, 161.
- Lee, J.H.; Kim, Y.T. Cloning and Characterization of the Astaxanthin Biosynthesis gene Cluster from the Marine Bacterium Paracoccus haeundaensis. Gene 2006, 370, 86–95.
- Lee, J.H.; Hwang, Y.M.; Baik, K.S.; Choi, K.S.; Ka, J.-O.; Seong, C.N. Mesoflavibacter aestuarii sp. nov., a Zeaxanthin Producing Marine Bacterium Isolated from Seawater. Int. J. Syst. Evol. Microbiol. 2014, 64, 1932–1937.
- Hameed, A.; Shahina, M.; Lin, S.-Y.; Lai, W.-A.; Hsu, Y.-H.; Liu, Y.-C.; Young, C.-C. Aquibacter zeaxanthinifaciens gen. nov., sp. nov., a Zeaxanthin-Producing Bacterium of the Family Flavobacteriaceae Isolated from Surface Seawater, and Emended Descriptions of the Genera Aestuariibaculum and Gaetbulibacter. Int. J. Syst. Evol. Microbiol. 2014, 64, 138–145.
- Asker, D.; Beppu, T.; Ueda, K. Zeaxanthinibacter enoshimensis gen. nov., sp. nov., a Novel Zeaxanthin-Producing Marine Bacterium of the Family Flavobacteriaceae, Isolated from Seawater Off Enoshima Island, Japan. Int. J. Syst. Evol. Microbiol. 2007, 57, 837–843.
- Shahina, M.; Hameed, A.; Lin, S.-Y.; Lee, R.-J.; Lee, M.-R.; Young, C.-C. Gramella planctonica sp. nov., a Zeaxanthin-Producing Bacterium Isolated from Surface Seawater, and Emended Descriptions of Gramella aestuarii and Gramella echinicola. Antonie van Leeuwenhoek 2014, 105, 771–779.
- Asker, D.; Beppu, T.; Ueda, K. Mesoflavibacter zeaxanthinifaciens gen. nov., sp. nov., a Novel Zeaxanthin Producing Marine Bacterium of the Family Flavobacteriaceae. Syst. Appl. Microbiol. 2007, 30, 291–296.
- Sowmya, R.; Sachindra, N.M. Carotenoid Production by Formosa sp. KMW, Marine Bacteria of Flavobacteriaceae Family: Influence of Culture Conditions and Nutrient Composition. Biocatal. Agric. Biotechnol. 2015, 4, 559–567.
- Thawornwiriyanun, P.; Tanasupawat, S.; Dechsakulwatana, C.; Techkarnjanaruk, S.; Suntornsuk, W. Identification of Newly Zeaxanthin-Producing Bacteria Isolated from Sponges in the Gulf of Thailand and Their Zeaxanthin Production. Appl. Biochem. Biotechnol. 2012, 167, 2357–2368.
- Hameed, A.; Arun, A.B.; Ho, H.-P.; Chang, C.-M.J.; Rekha, P.D.; Lee, M.-R.; Young, C.-C. Supercritical Carbon Dioxide Micronization of Zeaxanthin from Moderately Thermophilic Bacteria Muricauda lutaonensis CC-HSB-11T. J. Agric. Food Chem. 2011, 59, 4119–4124.
- Seto, R.; Takaichi, S.; Kurihara, T.; Kishi, R.; Honda, M.; Takenaka, S.; Tsukatani, Y.; Madigan, M.T.; Wang-Otomo, Z.Y.; Kimura, Y. Lycopene-Family Carotenoids Confer Thermostability on Photocomplexes from a New Thermophilic Purple Bacterium. Biochemistry 2020, 59, 2351–2358.
- Ramanathan, G.; Ramalakshmi, P. Studies on Efficacy of Marine Bacterium Salinicoccus roseus Pigment for Their Bioactive Potential. Eur. J. Biomed. Pharm. Sci. 2017, 4, 330–334.
- Montero, O.; Macías-Sánchez, M.D.; Lama, C.M.; Lubián, L.M.; Mantell, C.; Rodríguez, M.; De la Ossa, E.M. Supercritical CO2 Extraction of â-Carotene from a Marine Strain of the Cyanobacterium Synechococcus Species. J. Agric. Food Chem. 2005, 53, 9701–9707.
- Hamidi, M.; Kozani, P.S.; Kozani, P.S.; Pierre, G.; Michaud, P.; Delattre, C. Marine Bacteria Versus Microalgae: Who Is the Best for Biotechnological Production of Bioactive Compounds with Antioxidant Properties and Other Biological Applications? Mar. Drugs. 2020, 18, 28.
- Sibero, M.T.; Bachtiarini, T.U.; Trianto, A.; Lupita, A.H.; Sari, D.P.; Igarashi, Y.; Harunari, E.; Sharma, A.R.; Radjasa, O.K.; Sabdono, A. Characterization of a Yellow Pigmented Coral-Associated Bacterium Exhibiting Anti-Bacterial Activity against Multidrug Resistant (MDR) Organism. Egypt. J. Aquat. Res. 2018, 45, 81–87.
- Teramoto, M.; Nishijima, M. Flavicella marina gen. nov., sp. nov., a Carotenoid-Producing Bacterium from Surface Seawater. Int. J. Syst. Evol. Microbiol. 2015, 65, 799–804.
- Loh, W.L.C.; Huang, K.-C.; Ng, H.S.; Lan, J.C.-W. Exploring the Fermentation Characteristics of a Newly Isolated Marine Bacteria Strain, Gordonia terrae TWRH01 for Carotenoids Production. J. Biosci. Bioeng. 2020, 130, 187–194.
- Saha, S.; Thavasi, T.R.; Jayalakshmi, S. Phenazine Pigments from Pseudomonas aeruginosa and Their Application as Antibacterial Agent and Food Colourants. Res. J. Microbiol. 2008, 3, 122–128.
- Fulton, J.M.; Arthur, M.A.; Freeman, K.H. Subboreal Aridity and Scytonemin in the Holocene Black Sea. Org. Geochem. 2012, 49, 47–55.
- Soule, T.; Palmer, K.; Gao, Q.; Potrafka, R.M.; Stout, V.; Garcia-Pichel, F. A Comparative Genomics Approach to Understanding the Biosynthesis of The sunscreen Scytonemin in Cyanobacteria. BMC Genom. 2009, 10, 336.
- Thøgersen, M.S.; Delpin, M.W.; Melchiorsen, J.; Kilstrup, M.; Månsson, M.; Bunk, B.; Spröer, C.; Overmann, J.; Nielsen, K.F.; Gram, L. Production of the Bioactive Compounds Violacein and Indolmycin Is Conditional in a maeA Mutant of Pseudoalteromonas luteoviolacea S4054 Lacking the Malic Enzyme. Front. Microbiol. 2016, 7.
- Aye, A.M.; Bonnin-Jusserand, M.; Brian-Jaisson, F.; Ortalo-Magne, A.; Culioli, G.; Nevry, R.K.; Rabah, N.; Blache, Y.; Molmeret, M. Modulation of Violacein Production and Phenotypes Associated with Biofilm by Exogenous Quorum Sensing N-acylhomoserine Lactones in the Marine Bacterium Pseudoalteromonas ulvae TC14. Microbiology 2015, 161, 2039–2052.
- Dang, H.T.; Yotsumoto, K.; Enomoto, K. Draft Genome Sequence of Violacein-Producing Marine Bacterium Pseudoalteromonas sp. 520P1. Genome Announc. 2014, 2.
- Ballestriero, F.; Daim, M.; Penesyan, A.; Nappi, J.; Schleheck, D.; Bazzicalupo, P.; Schiavi, E.D.; Egan, S. Antinematode Activity of Violacein and the Role of the Insulin/IGF-1 Pathway in Controlling Violacein Sensitivity in Caenorhabditis elegans. PLoS ONE 2014, 9, e109201.
- Wu, Y.-H.; Cheng, H.; Xu, L.; Jin, X.-B.; Wang, C.-S.; Xu, X.-W. Physiological and Genomic Features of a Novel Violacein-Producing Bacterium Isolated from Surface Seawater. PLoS ONE 2017, 12, e0179997.
- Yada, S.; Wang, Y.; Zou, Y.; Nagasaki, K.; Hosokawa, K.; Osaka, I.; Arakawa, R.; Enomoto, K. Isolation and Characterization of Two Groups of Novel Marine Bacteria Producing Violacein. Mar. Biotechnol. 2008, 10, 128–132.
- Hakvåg, S.; Fjærvik, E.; Klinkenberg, G.; Borgos, S.E.F.; Josefsen, K.D.; Ellingsen, T.E.; Zotchev, S.B. Violacein-Producing Collimonas sp. from the Sea Surface Microlayer of Coastal Waters in Trøndelag, Norway. Mar. Drugs 2009, 7, 576–588.
- Vasanthabharathi, V.; Lakshminarayanan, R.; Jayalakshmi, S. Melanin Production from Marine Streptomyces. Afr. J. Biotechnol. 2011, 10, 11224–11234.
- Tarangini, K.; Mishra, S. Production, Characterization and Analysis of Melanin from Isolated Marine Pseudomonas sp. Using Vegetable Waste. Res. J. Eng. Sci. 2013, 2, 40–46.
- Lucas-Elío, P.; Goodwin, L.; Woyke, T.; Pitluck, S.; Nolan, M.; Kyrpides, N.C.; Detter, J.C.; Copeland, A.; Teshima, H.; Bruce, D.; et al. The Genomic Standards Consortium Complete Genome Sequence of the Melanogenic Marine Bacterium Marinomonas mediterranea Type Strain (MMB-1T). Stand. Genom. Sci. 2012, 6, 63–73.
- Manirethan, V.; Raval, K.; Balakrishnan, R.M. Adsorptive Removal of Trivalent and Pentavalent Arsenic from Aqueous Solutions Using Iron and Copper Impregnated Melanin Extracted from the Marine Bacterium Pseudomonas stutzeri. Environ. Pollut. 2019, 257, 113576.
- Kurian, N.K.; Nair, H.P.; Bhat, S.G. Evaluation of Anti-Inflammatory Property of Melanin from Marine Bacillus spp. BTCZ31. Asian J. Pharm. Clin. Res. 2015, 8, 251–255.
- Sivaperumal, P.; Kamala, K.; Rajaram, R.; Mishra, S.S. Melanin from Marine Streptomyces sp. (MVCS13) with Potential Effect against Ornamental Fish Pathogens of Carassius auratus. Biocatal. Agric. Biotechnol. 2014, 3, 134–141.
- Kurian, N.K.; Bhat, S.G. Photoprotection and Anti-Inflammatory Properties of Non–Cytotoxic Melanin from Marine Isolate Providencia rettgeri Strain BTKKS1. Biosci. Biotechnol. Res. Asia 2017, 14, 1475–1484.
- Kurian, N.K.; Nair, H.P.; Bhat, S.G. Characterization of Melanin Producing Bacteria Isolated from 96m depth Arabian Sea Sediments. Res. J. Biotechnol. 2019, 14, 64–71.
- Kamarudheen, N.; Naushad, T.; Rao, K.V.B. Biosynthesis, Characterization and Antagonistic Applications of Extracellular Melanin Pigment from Marine Nocardiopsis Sps. Indian J. Pharm. Educ. Res. 2019, 53, 112–120.
- Kurian, N.K.; Bhat, S.G. Food, Cosmetic and Biological Applications of Characterized DOPA-Melanin from Vibrio alginolyticus strain BTKKS3. Appl. Biol. Chem. 2018, 61, 163–171.
- Sakai-Kawada, F.E.; Ip, C.G.; Hagiwara, K.A.; Awaya, J.D. Biosynthesis and Bioactivity of Prodiginine Analogs in Marine Bacteria, Pseudoalteromonas: A Mini Review. Front. Microbiol. 2019, 10, 1715.
- Picott, K.J.; Deichert, J.A.; De Kemp, E.M.; Schatte, G.; Sauriol, F.; Ross, A.C. Isolation and Characterization of Tambjamine MYP1, a Macrocyclic Tambjamine Analogue from Marine Bacterium Pseudoalteromonas citrea. MedChemComm 2019, 10, 478–483.
- Lee, J.C.; Kim, Y.-S.; Park, S.; Kim, J.; Kang, S.-J.; Lee, M.-H.; Ryu, S.; Choi, J.M.; Oh, T.-K.; Yoon, J.-H. Exceptional Production of Both Prodigiosin and Cycloprodigiosin as Major Metabolic Constituents by a Novel Marine Bacterium, Zooshikella rubidus S1-1. Appl. Environ. Microbiol. 2011, 77, 4967–4973.
- Karbalaei-Heidari, H.R.; Partovifar, M.; Memarpoor-Yazdi, M. Evaluation of the Bioactive Potential of Secondary Metabolites Produced by a New Marine Micrococcus Species Isolated from the Persian Gulf. Avicenna J. Med. Biotechnol. 2020, 12, 61–65.
- Atalah, J.; Blamey, L.; Muñoz‑Ibacache, S.; Gutierrez, F.; Urzua, M.; Encinas, M.V.; Páez, M.; Sun, J.; Blamey, J.M. Isolation and Characterization of Violacein from an Antarctic Iodobacter: A Non‑Pathogenic Psychrotolerant Microorganism. Extremophiles 2019, 24, 43–52.
- Srilekha, V.; Krishna, G.; Srinivas, V.S.; Charya, M.A.S. Antimicrobial evaluation of bioactive pigment from Salinicoccus sp. isolated from Nellore sea coast. Int. J. Biotechnol. Biochem. 2017, 13, 211–217.
- Sasidharan, A.; Sasidharan, N.K.; Amma, D.B.N.S.; Vasu, R.K.; Nataraja, A.V.; Bhaskaran, K. Antifungal Activity of Violacein Purified from a Novel Strain of Chromobacterium sp. NIIST (MTCC 5522). J. Microbiol. 2015, 53, 694–701.
- Fehér, D.; Barlow, R.S.; Lorenzo, P.S.; Hemscheidt, T.K. A 2-Substituted Prodiginine, 2-(p-Hydroxybenzyl) Prodigiosin, from Pseudoalteromonas rubra. J. Nat. Prod. 2008, 71, 1970–1972.
- Patil, S.; Paradeshi, J.; Chaudhari, B. Anti-Melanoma and UV-B Protective Effect of Microbial Pigment Produced by Marine Pseudomonas aeruginosa GS-33. Nat. Prod. Res. 2016, 30, 2835–2839.
- Shindo, K.; Kikuta, K.; Suzuki, A.; Katsuta, A.; Kasai, H.; Yasumoto-Hirose, M.; Matsuo, Y.; Misawa, N.; Takaichi, S. Rare Carotenoids, (3R)-saproxanthin and (3R,2′S)-myxol, Isolated from Novel Marine Bacteria (Flavobacteriaceae) and Their Antioxidative Activities. Appl. Microbiol. Biotechnol. 2007, 74, 1350–1357.
- Prabhu, S.; Rekha, P.D.; Young, C.C.; Hameed, A.; Lin, S.-Y.; Arun, A.B. Zeaxanthin Production by Novel Marine Isolates from Coastal Sand of India and Its Antioxidant Properties. Appl. Biochem. Biotechnol. 2013, 171, 817–831.
- Kumar, G.; Sahu, N.; Reddy, G.N.; Prasad, R.B.N.; Nagesh, N.; Kamal, A. Production of Melanin Pigment from Pseudomonas stutzeri Isolated from Red Seaweed Hypneamusci formis. Lett. Appl. Microbiol. 2013, 57, 295–302.
- Renugadevi, K.; Nachiyar, C.V.; Sowmiya, P.; Sunkar, S. Antioxidant Activity of Phycocyanin Pigment Extracted from Marine Filamentous Cyanobacteria Geitlerinema sp TRV57. Biocatal. Agric. Biotechnol. 2018, 16, 237–242.
- Srinivasan, M.; Keziah, S.M.; Hemalatha, M.; Devi, C.S. Pigment from Streptomyces bellus MSA1 Isolated from Marine Sediments. IOP Conf. Ser. Mater. Sci. Eng. 2017, 263.
- Silva, T.R.; Tavares, R.S.N.; Canela-Garayoa, R.; Eras, J.; Rodrigues, M.V.N.; Neri-Numa, I.A.; Pastore, G.M.; Rosa, L.H.; Schultz, J.A.A.; Debonsi, H.M.; et al. Chemical Characterization and Biotechnological Applicability of Pigments Isolated from Antarctic Bacteria. Mar. Biotechnol. 2019, 21, 416–429.
- Metwally, R.A.; Abeer, A.; El-Sikaily, A.; El-Sersy, N.A.; Ghozlan, H.; Sabry, S. Biological Activity of Prodigiosin from Serratia rubidaea RAM_Alex. Res. J. Biotechnol. 2019, 14, 100.
- Hegazy, G.E.; Abu-Serie, M.M.; Abo-Elela, G.M.; Ghozlan, H.; Sabry, S.A.; Soliman, N.A.; Abdel-Fattah, Y.R. In Vitro Dual (Anticancer and Antiviral) Activity of the Carotenoids Produced by Haloalkaliphilic Archaeon Natrialba sp. M6. Sci. Rep. 2020, 10, 5986.
- Dharmaraj, S.; Ashokkumar, B.; Dhevendaran, K. Food-Grade Pigments from Streptomyces sp. Isolated from the Marine Sponge Callyspongia diffusa. Food Res. Int. 2009, 42, 487–492.
- Ramesh, C.; Vinithkumar, N.V.; Kirubagaran, R.; Venil, C.K.; Dufossé, L. Applications of Prodigiosin Extracted from Marine Red Pigmented Bacteria Zooshikella sp. and Actinomycete Streptomyces sp. Microorganisms 2020, 8, 556.
- Alihosseini, F.; Ju, K.-S.; Lango, J.; Hammock, B.D.; Sun, G. Antibacterial Colorants: Characterization of Prodiginines and Their Applications on Textile Materials. Biotechnol. Prog. 2008, 24, 742–747.
- Anti-Bacterial Fabric Holds Promise for Fighting Superbug. Science Daily. Ulsan National Institute of Science and Technology (UNIST). Available online: https://www.sciencedaily.com/releases/2016/03/160308091646.htm (accessed on 8 March 2020).
- Michael, R. New Antibiotic Dye May Help Prevent Infectious Diseases. Contagion Live. Available online: https://www.contagionlive.com/news/new-antibiotic-dye-may-help-prevent-infectious-diseases (accessed on 4 April 2020).
- Krishna, J.G.; Jacob, A.; Kurian, P.; Elyas, K.K.; Chandrasekaran, M. Marine Bacterial Prodigiosin as Dye for Rubber Latex, Polymethyl Methacrylate Sheets and Paper. Afr. J. Biotechnol. 2013, 12, 2266–2269.
- Derikvand, P.; Llewellyn, C.A.; Purton, S. Cyanobacterial Metabolites as a Source of Sunscreens and Moisturizers: A Comparison with Current Synthetic Compounds. Eur. J. Phycol. 2017, 52, 43–56.
- Consumption Value of Cosmetics and Personal Care in Europe in 2018, by Country (in Million Euros). Available online: https://www.statista.com/statistics/382100/european-cosmetics-market-volume-by-country/ (accessed on 24 June 2020).
- Poulose, N.; Sajayan, A.; Ravindran, A.; Sreechitra, T.; Vardhan, V.; Selvin, J.; Kiran, G.S. Photoprotective Effect of Nanomelanin-Seaweed Concentrate in Formulated Cosmetic Cream: With Improved Antioxidant and Wound Healing Properties. J. Photochem. Photobiol. B Biol. 2020, 205.
- Wang, Z.; Tschirhart, T.; Schultzhaus, Z.; Kelly, E.E.; Chen, A.; Oh, E.; Nag, O.; Glaser, E.R.; Kim, E.; Lloyd, P.F.; et al. Characterization and Application of Melanin Produced by the Fast-Growing Marine Bacterium Vibrio natriegens Through Heterologous Biosynthesis. Appl. Environ. Microbiol. 2019, 86.
- Priya, K.A.; Satheesh, S.; Balasubramaniem, A.K.; Varalakshmi, P.; Gopal, S.; Natesan, S. Antifouling Activity of Prodigiosin from Estuarine Isolate of Serratia marcescens CMST 07. In Microbiological Research In Agroecosystem Management, 1st ed.; Velu, R.K., Ed.; Springer: New Delhi, India, 2013; pp. 11–21.
- Zeng, Z.; Guo, X.-P.; Cai, X.; Wang, P.; Li, B.; Yang, J.-L.; Wang, X. Pyomelanin from Pseudoalteromonas lipolytica Reduces Biofouling. Microbiol. Biotechnol. 2017, 10, 1718–1731.
- Hernández-Velasco, P.; Morales-Atilano, I.; Rodríguez-Delgado, M.; Rodríguez-Delgado, J.M.; Luna-Moreno, D.; Ávalos-Alanís, F.G.; Villarreal-Chiu, J.F. Photoelectric Evaluation of Dye-Sensitized Solar Cells Based on Prodigiosin Pigment Derived from Serratia marcescens 11E. Dyes Pigment. 2020, 177, 108278.
