Chronic granulomatous disease (CGD) is a primary immunodeficiency caused by a defect in the phagocytic function of the innate immune system owing to mutations in genes encoding the five subunits of the nicotinamide adenine dinucleotide phosphatase (NADPH) oxidase enzyme complex. The most common microorganisms observed in the patients with CGD are Staphylococcus aureus, Aspergillus spp., Candida spp., Nocardia spp., Burkholderia spp., Serratia spp., and Salmonella spp. Antibacterial prophylaxis with trimethoprim-sulfamethoxazole, antifungal prophylaxis usually with itraconazole, and interferon gamma immunotherapy have been successfully used in reducing infection in CGD. Haematopoietic stem cell transplantation (HCT) have been successfully proven to be the treatment of choice in patients with CGD.
- chronic granulomatous disease
- microorganisms
- neutrophils
- antimicrobials
1. Introduction
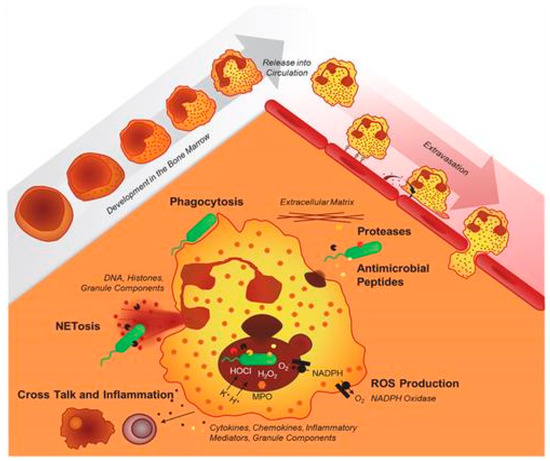
2. Pathogenesis
3. Clinical Manifestations
3.1. CGD-Related Inflammatory Responses
3.2. Hemophagocytic Lymphohistiocytosis (HLH)
4. CGD Incidence
5. Granulomas
6. Pathogens and CGD-Related Infectious Diseases
6.1. Pathogens
6.2. CGD-Related Infectious Diseases
7. Laboratory Diagnosis
7.1. Neutrophil-Function Testing
7.2. Nitroblue Tetrazolium (NBT) Reduction Test
7.3. Flow Cytometric Dihydrorhodamine Assay
7.4. Luminol-Enhanced Chemiluminescence Assay
7.5. Genetic Testing
8. Management of CGD
8.1. Haematopoietic Stem Cell Transplantation (HSCT/HCT)
8.2. Drug-Based Treatment
8.3. Gene Therapy
9. Conclusions
This entry is adapted from the peer-reviewed paper 10.3390/microorganisms11092233
References
- Bridges, R.A.; Berendes, H.; Good, R.A. A Fatal Granulomatous Disease of Childhood; the Clinical, Pathological, and Laboratory Features of a New Syndrome. AMA J. Dis. Child. 1959, 97, 387–408.
- O’Shea, P.A. Chronic Granulomatous Disease of Childhood. Perspect. Pediatr. Pathol. 1982, 7, 237–258.
- Good, R.A.; Quie, P.G.; Windhorst, D.B.; Page, A.R.; Rodey, G.E.; White, J.; Wolfson, J.J.; Holmes, B.H. Fatal (chronic) Granulomatous Disease of Childhood: A Hereditary Defect of Leukocyte Function. Semin. Hematol. 1968, 5, 215–254.
- Anjani, G.; Vignesh, P.; Joshi, V.; Shandilya, J.K.; Bhattarai, D.; Sharma, J.; Rawat, A. Recent Advances in Chronic Granulomatous Disease. Genes Dis. 2020, 7, 84–92.
- Roos, D. Chronic Granulomatous Disease. Br. Med. Bull. 2016, 118, 50–63.
- Mollin, M.; Beaumel, S.; Vigne, B.; Brault, J.; Roux-Buisson, N.; Rendu, J.; Barlogis, V.; Catho, G.; Dumeril, C.; Fouyssac, F.; et al. Clinical, Functional and Genetic Characterization of 16 Patients Suffering from Chronic Granulomatous Disease Variants—Identification of 11 Novel Mutations in CYBB. Clin. Exp. Immunol. 2021, 203, 247–266.
- Dinauer, M.C. Inflammatory Consequences of Inherited Disorders Affecting Neutrophil Function. Blood 2019, 133, 2130–2139.
- Kruger, P.; Saffarzadeh, M.; Weber, A.N.R.; Rieber, N.; Radsak, M.; von Bernuth, H.; Benarafa, C.; Roos, D.; Skokowa, J.; Hartl, D. Neutrophils: Between Host Defence, Immune Modulation, and Tissue Injury. PLoS Pathog. 2015, 11, e1004651.
- Rider, N.L.; Jameson, M.B.; Creech, C.B. Chronic Granulomatous Disease: Epidemiology, Pathophysiology, and Genetic Basis of Disease. J. Pediatr. Infect. Dis. Soc. 2018, 7, S2–S5.
- Mauch, L.; Lun, A.; O’Gorman, M.R.G.; Harris, J.S.; Schulze, I.; Zychlinsky, A.; Fuchs, T.; Oelschlägel, U.; Brenner, S.; Kutter, D.; et al. Chronic Granulomatous Disease (CGD) and Complete Myeloperoxidase Deficiency Both Yield Strongly Reduced Dihydrorhodamine 123 Test Signals but Can Be Easily Discerned in Routine Testing for CGD. Clin. Chem. 2007, 53, 890–896.
- Roos, D. Chronic Granulomatous Disease. In NADPH Oxidases: Methods and Protocols; Knaus, U.G., Leto, T.L., Eds.; Springer: New York, NY, USA, 2019; pp. 531–542. ISBN 9781493994243.
- Yu, H.-H.; Yang, Y.-H.; Chiang, B.-L. Chronic Granulomatous Disease: A Comprehensive Review. Clin. Rev. Allergy Immunol. 2021, 61, 101–113.
- Valentine, G.; Thomas, T.A.; Nguyen, T.; Lai, Y.-C. Chronic Granulomatous Disease Presenting as Hemophagocytic Lymphohistiocytosis: A Case Report. Pediatrics 2014, 134, e1727–e1730.
- Marciano, B.E.; Spalding, C.; Fitzgerald, A.; Mann, D.; Brown, T.; Osgood, S.; Yockey, L.; Darnell, D.N.; Barnhart, L.; Daub, J.; et al. Common Severe Infections in Chronic Granulomatous Disease. Clin. Infect. Dis. 2015, 60, 1176–1183.
- Curnutte, J.T.; Scott, P.J.; Mayo, L.A. Cytosolic Components of the Respiratory Burst Oxidase: Resolution of Four Components, Two of Which Are Missing in Complementing Types of Chronic Granulomatous Disease. Proc. Natl. Acad. Sci. USA 1989, 86, 825–829.
- Wolach, B.; Gavrieli, R.; de Boer, M.; Gottesman, G.; Ben-Ari, J.; Rottem, M.; Schlesinger, Y.; Grisaru-Soen, G.; Etzioni, A.; Roos, D. Chronic Granulomatous Disease in Israel: Clinical, Functional and Molecular Studies of 38 Patients. Clin. Immunol. 2008, 129, 103–114.
- Fattahi, F.; Badalzadeh, M.; Sedighipour, L.; Movahedi, M.; Fazlollahi, M.R.; Mansouri, S.D.; Khotaei, G.T.; Bemanian, M.H.; Behmanesh, F.; Hamidieh, A.A.; et al. Inheritance Pattern and Clinical Aspects of 93 Iranian Patients with Chronic Granulomatous Disease. J. Clin. Immunol. 2011, 31, 792–801.
- Kutukculer, N.; Aykut, A.; Karaca, N.E.; Durmaz, A.; Aksu, G.; Genel, F.; Pariltay, E.; Cogulu, Ö.; Azarsız, E. Chronic Granulamatous Disease: Two Decades of Experience from a Paediatric Immunology Unit in a Country with High Rate of Consangineous Marriages. Scand. J. Immunol. 2019, 89, e12737.
- Wolach, B.; Gavrieli, R.; de Boer, M.; van Leeuwen, K.; Berger-Achituv, S.; Stauber, T.; Ben Ari, J.; Rottem, M.; Schlesinger, Y.; Grisaru-Soen, G.; et al. Chronic Granulomatous Disease: Clinical, Functional, Molecular, and Genetic Studies. The Israeli Experience with 84 Patients. Am. J. Hematol. 2017, 92, 28–36.
- van den Berg, J.M.; van Koppen, E.; Ahlin, A.; Belohradsky, B.H.; Bernatowska, E.; Corbeel, L.; Español, T.; Fischer, A.; Kurenko-Deptuch, M.; Mouy, R.; et al. Chronic Granulomatous Disease: The European Experience. PLoS ONE 2009, 4, e5234.
- Slack, M.A.; Thomsen, I.P. Prevention of Infectious Complications in Patients With Chronic Granulomatous Disease. J. Pediatr. Infect. Dis. Soc. 2018, 7, S25–S30.
- Alimchandani, M.; Lai, J.-P.; Aung, P.P.; Khangura, S.; Kamal, N.; Gallin, J.I.; Holland, S.M.; Malech, H.L.; Heller, T.; Miettinen, M.; et al. Gastrointestinal Histopathology in Chronic Granulomatous Disease: A Study of 87 Patients. Am. J. Surg. Pathol. 2013, 37, 1365–1372.
- Winkelstein, J.A.; Marino, M.C.; Johnston, R.B., Jr.; Boyle, J.; Curnutte, J.; Gallin, J.I.; Malech, H.L.; Holland, S.M.; Ochs, H.; Quie, P.; et al. Chronic Granulomatous Disease. Report on a National Registry of 368 Patients. Medicine 2000, 79, 155–169.
- Dotis, J.; Pana, Z.D.; Roilides, E. Non-Aspergillus Fungal Infections in Chronic Granulomatous Disease. Mycoses 2013, 56, 449–462.
- Kobayashi, S.; Murayama, S.; Takanashi, S.; Takahashi, K.; Miyatsuka, S.; Fujita, T.; Ichinohe, S.; Koike, Y.; Kohagizawa, T.; Mori, H.; et al. Clinical Features and Prognoses of 23 Patients with Chronic Granulomatous Disease Followed for 21 Years by a Single Hospital in Japan. Eur. J. Pediatr. 2008, 167, 1389–1394.
- Mortaz, E.; Sarhifynia, S.; Marjani, M.; Moniri, A.; Mansouri, D.; Mehrian, P.; van Leeuwen, K.; Roos, D.; Garssen, J.; Adcock, I.M.; et al. An Adult Autosomal Recessive Chronic Granulomatous Disease Patient with Pulmonary Aspergillus terreus Infection. BMC Infect. Dis. 2018, 18, 552.
- Roos, D.; de Boer, M. Molecular Diagnosis of Chronic Granulomatous Disease. Clin. Exp. Immunol. 2014, 175, 139–149.
- Chen, Y.; Junger, W.G. Measurement of Oxidative Burst in Neutrophils. Methods Mol. Biol. 2012, 844, 115–124.
- Marrocco, I.; Altieri, F.; Peluso, I. Measurement and Clinical Significance of Biomarkers of Oxidative Stress in Humans. Oxid. Med. Cell. Longev. 2017, 2017, 6501046.
- Verbsky, J.W.; Routes, J.M. Recurrent Fever, Immune Deficiency, and Autoinflammatory Disorders. In Nelson Pediatric Symptom-Based Diagnosis: Common Diseases and Their Mimics; Elsevier: Amsterdam, The Netherlands, 2023; pp. 1015–1046.e1. ISBN 9780323761741.
- Jirapongsananuruk, O.; Malech, H.L.; Kuhns, D.B.; Niemela, J.E.; Brown, M.R.; Anderson-Cohen, M.; Fleisher, T.A. Diagnostic Paradigm for Evaluation of Male Patients with Chronic Granulomatous Disease, Based on the Dihydrorhodamine 123 Assay. J. Allergy Clin. Immunol. 2003, 111, 374–379.
- Kuhns, D.B.; Alvord, W.G.; Heller, T.; Feld, J.J.; Pike, K.M.; Marciano, B.E.; Uzel, G.; DeRavin, S.S.; Priel, D.A.L.; Soule, B.P.; et al. Residual NADPH Oxidase and Survival in Chronic Granulomatous Disease. N. Engl. J. Med. 2010, 363, 2600–2610.
- Blancas-Galicia, L.; Santos-Chávez, E.; Deswarte, C.; Mignac, Q.; Medina-Vera, I.; León-Lara, X.; Roynard, M.; Scheffler-Mendoza, S.C.; Rioja-Valencia, R.; Alvirde-Ayala, A.; et al. Genetic, Immunological, and Clinical Features of the First Mexican Cohort of Patients with Chronic Granulomatous Disease. J. Clin. Immunol. 2020, 40, 475–493.
- Sanabria, D.; Giménez, V.; de Cuéllar, C.M.; Carpinelli, M.; Benegas, S.; Insaurralde, S. Enfermedad Granulomatosa Crónica. Diagnóstico Mediante El Ensayo de Dihidrorodamina. Rev. Chil. Pediatría 2020, 91, 19–26.
- Ang, E.Y.; Soh, J.Y.; Liew, W.K.; Chan, K.W.; Thoon, K.C.; Chong, C.Y.; Lau, Y.L.; Lee, B.W. Reliability of Acute Illness Dihydrorhodamine-123 Testing for Chronic Granulomatous Disease. Clin. Lab. 2013, 59, 203–206.
- Lai, B.; Bernhardt, P.V.; Krömer, J.O. Cytochrome c Reductase Is a Key Enzyme Involved in the Extracellular Electron Transfer Pathway towards Transition Metal Complexes in Pseudomonas putida. ChemSusChem 2020, 13, 5308–5317.
- Yu, J.E.; Azar, A.E.; Chong, H.J.; Jongco, A.M., 3rd; Prince, B.T. Considerations in the Diagnosis of Chronic Granulomatous Disease. J. Pediatr. Infect. Dis. Soc. 2018, 7, S6–S11.
- Vowells, S.J.; Sekhsaria, S.; Malech, H.L.; Shalit, M.; Fleisher, T.A. Flow Cytometric Analysis of the Granulocyte Respiratory Burst: A Comparison Study of Fluorescent Probes. J. Immunol. Methods 1995, 178, 89–97.
- Leiding, J.W.; Holland, S.M. Chronic Granulomatous Disease; University of Washington: Seattle, WA, USA, 2022.
- Parvaneh, N.; Teimourian, S. Effectiveness of Nitroblue Tetrazolium (NBT) Test. Arch. Iran. Med. 2008, 11, 129–130; author reply 130.
- Güngör, T.; Teira, P.; Slatter, M.; Stussi, G.; Stepensky, P.; Moshous, D.; Vermont, C.; Ahmad, I.; Shaw, P.J.; Telles da Cunha, J.M.; et al. Reduced-Intensity Conditioning and HLA-Matched Haemopoietic Stem-Cell Transplantation in Patients with Chronic Granulomatous Disease: A Prospective Multicentre Study. Lancet 2014, 383, 436–448.
- Vowells, S.J.; Fleisher, T.A.; Sekhsaria, S.; Alling, D.W.; Maguire, T.E.; Malech, H.L. Genotype-Dependent Variability in Flow Cytometric Evaluation of Reduced Nicotinamide Adenine Dinucleotide Phosphate Oxidase Function in Patients with Chronic Granulomatous Disease. J. Pediatr. 1996, 128, 104–107.
- Kim, H.-Y.; Kim, H.-J.; Ki, C.-S.; Kim, D.W.; Yoo, K.H.; Kang, E.-S. Rapid Determination of Chimerism Status Using Dihydrorhodamine Assay in a Patient with X-Linked Chronic Granulomatous Disease Following Hematopoietic Stem Cell Transplantation. Ann. Lab. Med. 2013, 33, 288–292.
- Thiede, C. Diagnostic Chimerism Analysis after Allogeneic Stem Cell Transplantation: New Methods and Markers. Am. J. Pharmacogenom. 2004, 4, 177–187.
- Ríos, N.; Prolo, C.; Álvarez, M.N.; Piacenza, L.; Radi, R. Chapter 21—Peroxynitrite Formation and Detection in Living Cells. In Nitric Oxide, 3rd ed.; Ignarro, L.J., Freeman, B.A., Eds.; Academic Press: Cambridge, MA, USA, 2017; pp. 271–288. ISBN 9780128042731.
- Görlach, A.; Lee, P.L.; Roesler, J.; Hopkins, P.J.; Christensen, B.; Green, E.D.; Chanock, S.J.; Curnutte, J.T. A p47-Phox Pseudogene Carries the Most Common Mutation Causing p47-Phox- Deficient Chronic Granulomatous Disease. J. Clin. Investig. 1997, 100, 1907–1918.
- Kulkarni, M.; Hule, G.; de Boer, M.; van Leeuwen, K.; Kambli, P.; Aluri, J.; Gupta, M.; Dalvi, A.; Mhatre, S.; Taur, P.; et al. Approach to Molecular Diagnosis of Chronic Granulomatous Disease (CGD): An Experience from a Large Cohort of 90 Indian Patients. J. Clin. Immunol. 2018, 38, 898–916.
- Roos, D.; Kuhns, D.B.; Maddalena, A.; Roesler, J.; Lopez, J.A.; Ariga, T.; Avcin, T.; de Boer, M.; Bustamante, J.; Condino-Neto, A.; et al. Hematologically Important Mutations: X-Linked Chronic Granulomatous Disease (third Update). Blood Cells Mol. Dis. 2010, 45, 246–265.
- Yonkof, J.R.; Gupta, A.; Fu, P.; Garabedian, E.; Dalal, J. The United States Immunodeficiency Network Consortium Role of Allogeneic Hematopoietic Stem Cell Transplant for Chronic Granulomatous Disease (CGD): A Report of the United States Immunodeficiency Network. J. Clin. Immunol. 2019, 39, 448–458.
- Arnold, D.E.; Heimall, J.R. A Review of Chronic Granulomatous Disease. Adv. Ther. 2017, 34, 2543–2557.
- Chiesa, R.; Wang, J.; Blok, H.-J.; Hazelaar, S.; Neven, B.; Moshous, D.; Schulz, A.; Hoenig, M.; Hauck, F.; Al Seraihy, A.; et al. Hematopoietic Cell Transplantation in Chronic Granulomatous Disease: A Study of 712 Children and Adults. Blood 2020, 136, 1201–1211.
- Magnani, A.; Mahlaoui, N. Managing Inflammatory Manifestations in Patients with Chronic Granulomatous Disease. Paediatr. Drugs 2016, 18, 335–345.
- Yanagimachi, M.; Kato, K.; Iguchi, A.; Sasaki, K.; Kiyotani, C.; Koh, K.; Koike, T.; Sano, H.; Shigemura, T.; Muramatsu, H.; et al. Hematopoietic Cell Transplantation for Chronic Granulomatous Disease in Japan. Front. Immunol. 2020, 11, 1617.
- Marsh, R.A.; Leiding, J.W.; Logan, B.R.; Griffith, L.M.; Arnold, D.E.; Haddad, E.; Falcone, E.L.; Yin, Z.; Patel, K.; Arbuckle, E.; et al. Chronic Granulomatous Disease-Associated IBD Resolves and Does Not Adversely Impact Survival Following Allogeneic HCT. J. Clin. Immunol. 2019, 39, 653–667.
- Yi, E.S.; Choi, Y.B.; Lee, N.H.; Lee, J.W.; Sung, K.W.; Koo, H.H.; Kang, E.-S.; Kim, Y.-J.; Yoo, K.H. Allogeneic Hematopoietic Cell Transplantation in Patients with Primary Immunodeficiencies in Korea: Eleven-Year Experience in a Single Center. J. Clin. Immunol. 2018, 38, 757–766.
- Garcia-Eulate, R.; Hussain, N.; Heller, T.; Kleiner, D.; Malech, H.; Holland, S.; Choyke, P.L. CT and MRI of Hepatic Abscess in Patients with Chronic Granulomatous Disease. AJR Am. J. Roentgenol. 2006, 187, 482–490.
- Köker, M.Y.; Camcıoğlu, Y.; van Leeuwen, K.; Kılıç, S.Ş.; Barlan, I.; Yılmaz, M.; Metin, A.; de Boer, M.; Avcılar, H.; Patıroğlu, T.; et al. Clinical, Functional, and Genetic Characterization of Chronic Granulomatous Disease in 89 Turkish Patients. J. Allergy Clin. Immunol. 2013, 132, 1156–1163.e5.
- Ochs, H.D.; Edvard Smith, C.I.; Puck, J. Primary Immunodeficiency Diseases: A Molecular and Genetic Approach; Oxford University Press: Oxford, UK, 2007; ISBN 9780195147742.
- Conrad, A.; Neven, B.; Mahlaoui, N.; Suarez, F.; Sokol, H.; Ruemmele, F.M.; Rouzaud, C.; Moshous, D.; Lortholary, O.; Blanche, S.; et al. Infections in Patients with Chronic Granulomatous Disease Treated with Tumor Necrosis Factor Alpha Blockers for Inflammatory Complications. J. Clin. Immunol. 2021, 41, 185–193.
- Peixoto, A.; Coelho, R.; Maia, T.; Sarmento, A.; Magro, F.; Macedo, G. Chronic Granulomatous Disease Mimicking Colonic Crohn’s Disease Successfully Treated with Infliximab. ACG Case Rep. J. 2017, 4, e46.
- Jafarian, A.; Shokri, G.; Shokrollahi Barough, M.; Moin, M.; Pourpak, Z.; Soleimani, M. Recent Advances in Gene Therapy and Modeling of Chronic Granulomatous Disease. Iran. J. Allergy Asthma Immunol. 2019, 18, 131–142.
- Renga, G.; Oikonomou, V.; Moretti, S.; Stincardini, C.; Bellet, M.M.; Pariano, M.; Bartoli, A.; Brancorsini, S.; Mosci, P.; Finocchi, A.; et al. Thymosin β4 Promotes Autophagy and Repair via HIF-1α Stabilization in Chronic Granulomatous Disease. Life Sci. Alliance 2019, 2, e201900432.
