Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
The use of synthetic/artificial preservatives has become common and has not been widely accepted by consumers as they are aware of the fact that exposure to preservatives can lead to adverse effects on health, which is a major area of concern for researchers. Naturally occurring phenolic compounds appear to be extensively used as bio-preservatives to prolong the shelf life of the finished product.
- natural phenolic compounds
- preservatives
- antioxidant
- microbial activities
1. Introduction
Shelf life refers to the duration between manufacturing and expiry date, during which the product is expected to retain its original characteristics and remain acceptable for consumers as far as its quality is concerned. During this duration, the product is susceptible to chemical, biological, and physical deterioration, which ultimately degrade the qualitative characteristics of the product [1]. Therefore, preservatives have been extensively used in various pharmaceuticals, cosmetics, and food products to prevent them from deterioration [2][3]. Many factors are responsible for governing the mean life of the product such as the growth of microorganisms, heating, inappropriate temperature, long storage, change in moisture content, reaction with light and oxygen, fermentation, acidification, enzymatic changes, etc., which result in the loss of important properties of the finished product. Pharmaceutical preparation consists of a diverse range of structures and moieties that are susceptible to deterioration. Deterioration is the result of chemical reactions that occur between the various ingredients present in the formulation and the external environment. The deterioration of a product generally occurs during longer storage, affecting its stability, ultimately resulting in the product’s decline in its intended natural quality due to microbial contamination and rendering the product harmful to the consumer. In general, there are three processes by which the product degrades [4]. Chemical breakdown includes chemical incompatibilities, such as hydrolysis, oxidation, photolysis, polymerization, hydration, dehydration, and decarboxylation [5][6]. A change in temperature, particle size, evaporation, vaporization, efflorescence, hygroscope, deliquescence, etc. are all examples of physical degradation. Everywhere we look, there are microbes that assault the product once it is opened. The product starts losing its quality quickly on a microbiological level [7].
2. Preservatives and Their Needs
An antimicrobial preservative is a natural or artificial substance added to non-sterile products in favor of preventing their decomposition due to chemical, enzymatic, or microbial activities or any undesirable chemical changes, most probably caused by microbial growth and lipid oxidation [8][9] (Figure 1). The addition of preservatives is obligatory in the case of products containing water, or organic or inorganic compounds that are at the highest risk of contamination. Products such as creams, solutions, emulsions, suspensions, parental and eye drops, etc. are likely to be perishable, which may further lead to the spoilage of products and result in the loss of some essential properties [10][11]. Therefore, preservatives are meant to be introduced during the manufacturing process to keep the essential qualitative characteristics and organoleptic properties of the product intact by retarding degradation of the product formulation during its shelf life [12]. They keep the product fresher for a longer time so that no undesirable pathogen could attack and bring about unenviable changes in the finished product.
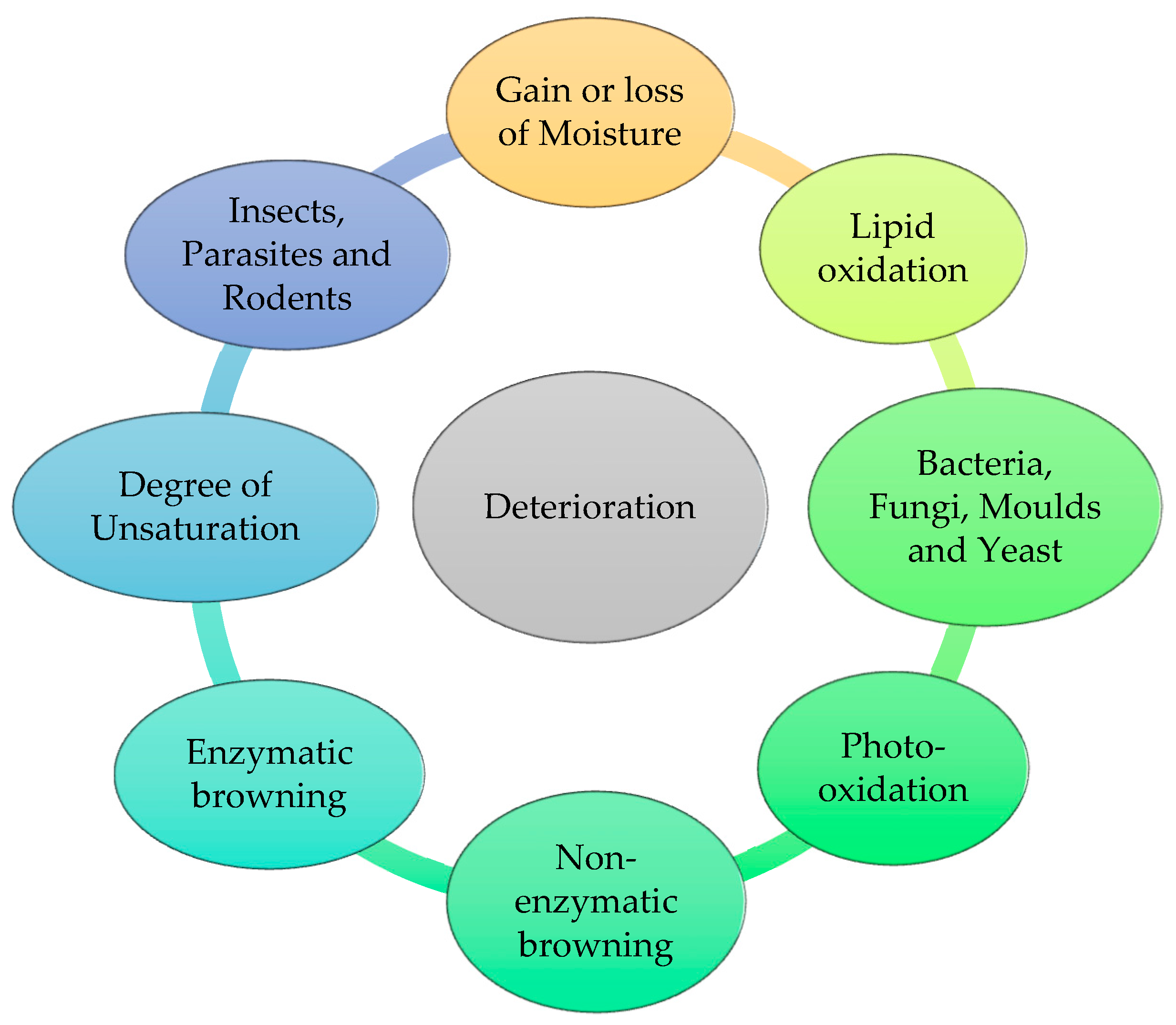
Figure 1. Causes of deterioration of the product.
3. Food Preservatives
Foodstuffs of various types such as raw food, junk food, fast food, organic food, whole food, processed or unprocessed food, and vegetarian or non-vegetarian food exist across the globe depending upon climatic and geographical conditions. Moreover, foodstuffs are subjected to storage in go downs or houses for handling emergencies [13]. Food products are perishable items with a short shelf life ranging from a few hours to a few days to a few months. For many years, food preservation has been a great challenge for the food industry in terms of ensuring quality, nutritional value, organoleptic properties, and safety [14][15]. Preservatives are generally added to decontaminate the food product and to ensure the stability and safety of the product. The consumer demands chemical-free, fresh, nutritionally rich, tasty, smells good, and ready-to-eat food, which appeals to the taste buds and has a prolonged shelf life too. This has prompted researchers/manufacturers to search and develop natural antimicrobial preservatives. Organic acids and their derivatives depicted in Table 1 are natural compounds that have been exploited by researchers as bio-preservatives for the last few decades. They are found to be inexpensive and effective at decontaminating the food product against the population of food pathogens and are generally recognized as safe (GRAS). Several studies reported that organic acids such as acetic, citric, lactic, propionic, benzoic, and sorbic acids possess an excellent antimicrobial potential, exhibiting a broad spectrum of activity against food pathogens such as bacteria fungi and yeast [16][17][18][19].
Table 1. List of existing food preservatives and their problems.
| Preservatives & Chemical Structure |
Toxic Effect | Food Products | References |
|---|---|---|---|
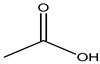 Acetic acid |
Breathing difficulty, swelling, irritation | Preserved fish, preserved meat, cooking oil, curry powder, processed cheese, instant puddings, butter, bread, mozzarella cheese, baby food, etc. | [20][21] |
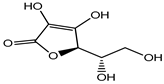 Ascorbic acid (Vitamin C) |
Nausea, vomiting, headache, heartburn and cramps | Fruit juices, jams, beer, soft drinks, cider, cereals, canned tuna, glaze for frozen fish, etc. | [22] |
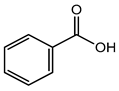 Benzoic acid |
Carcinogenic, irritation, metabolic acidosis, asthma, convulsions, etc. | Fruit juices, soft drinks, coffee extracts, pickles; pineapple marmalade with pectin; relishes; tomato paste; tomato pulp; tomato puree, etc. | [23][24][25] |
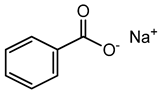 Sodium benzoate |
Allergy, asthma, skin rashes, hyperactivity, liver cirrhosis, Parkinson’s disease, etc. | Chayavanprash, fruit juices, fruit cocktails, tomato ketchup, pasta sauce, milk products, jams, soft drinks, salad dressings, pickles, margarine, etc. | [23][24][25][26] |
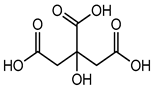 Citric acid |
Numbness, rapid weight gain, cramps, mood changes, severe stomach pain, diarrhea and convulsions, etc. | Fruit juices, soft drinks, meat, frozen food, chips, biscuits, ice creams, soda, wine, etc. | [27] |
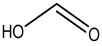 Formic acid |
Nausea, vomiting, dizziness, headache, skin allergy etc. | Livestock feed | [28] |
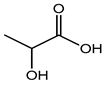 Lactic acid |
Brain fog, gas, and bloating | Yoghurt, olives, cucumber pickles, salad dressing, cheese, frozen desserts, soda, etc. | [29] |
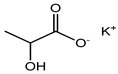 Potassium lactate |
Brain fog, gas, and bloating | Meat and poultry products | [29][30][31][32] |
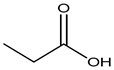 Propionic acid |
Corrosive, increase resistance to glucagon, norepinephrine, and insulin | Bakery products, gelatin, milk, and milk products such as cheese, yoghurt, etc. | [26][33] |
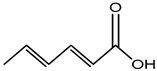 Sorbic acid |
Contact dermatitis and urticarial, etc. | Fruit juice, wine, jams, jellies, margarine, meat products, cakes, sauces, processed cheese, salads, etc. | [26] |
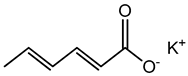 Potassium Sorbate |
Contact dermatitis and urticarial, etc. | Karela amla juice, Amla-Aloe vera juice with wheatgrass, milk products, etc. | [26] |
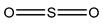 Sulphur dioxide/Sulphites 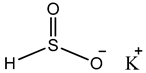 Potassium bisulfate |
Allergic reactions (nausea, skin irritation, asthma, eczema, diarrhea) |
Fruit juices, cider, wines, meat products, sausages, sweets, jams, jellies, glucose syrups, dry biscuits, dry mix of rasogollas, refined sugar, misri, jaggery, glucose syrup, potatoes, beverages, raisins, pickles, chutneys, etc. | [26] |
| Nitrites & Nitrates (Sodium & Potassium) |
Genotoxicity, carcinogenic (stomach and pancreatic cancer) | Meat products, hot dog, smoked fish, and many others | [26][34] |
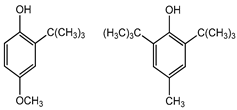 BHA BHT |
Endocrine disruptors, neurological problems, metabolic dysfunction, behavioral issues, and may cause cancer | Hot dogs, meat, potato chips, chewing gum, vegetable oils, bread, breakfast cereals, and many others | [35] |
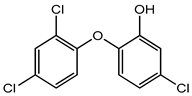 Triclosan |
Antibiotic resistance in bacteria, endocrine disruptor, skin sensitization, neuro-toxicity, reproductive and developmental toxicity, genotoxicity, carcinogenicity, phototoxicity, etc. | Active food packaging | [35][36][37] |
| Nisin | Nausea, pruritus, skin rash and vomiting, etc. | Prepacked coconut water, canned rasgulla, baking mixes containing egg, etc. | [38] |
| Dehyderoacetic acid (DHA) | Allergic contact dermatitis | Cheese, meat products, squash, etc. | [39] |
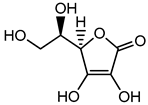 Erythorbic acid |
Hypersensitivity, nausea, vomiting, headache, diarrhea, heartburn, and cramps | Meat, frozen fruits and vegetables, beverages such as soft drinks, fruit juice, grape wine, beer, etc. | [40] |
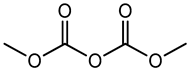 Dimethyl dicarbonate (DMDC) |
Carcinogenic | Non-alcoholic beverages such as sports drinks, energy drinks and non-carbonated drinks such as ice tea, etc. | [41][42][43] |
4. Natural Preservatives and Their Importance
Owing to their natural origin, bio-preservatives received lots of attention in recent years as they are much safer and more beneficial when compared to synthetic preservatives. Natural food preservatives are typically of plant, animal, and microbial origin. Natural products, derived from natural plant sources such as herbs, spices, and essential oils, are widely used to give aroma to beverages and mask the disagreeable odor of the constituents in addition to their preservative action (Figure 2) [44]. They are found to increase shelf life naturally by decreasing lipid oxidation. Some of the common conventional methods that can be used to preserve food are drying (natural and artificial), pickling (using salt or vinegar), freezing, high-pressure processing, and the edible coating technique [45].
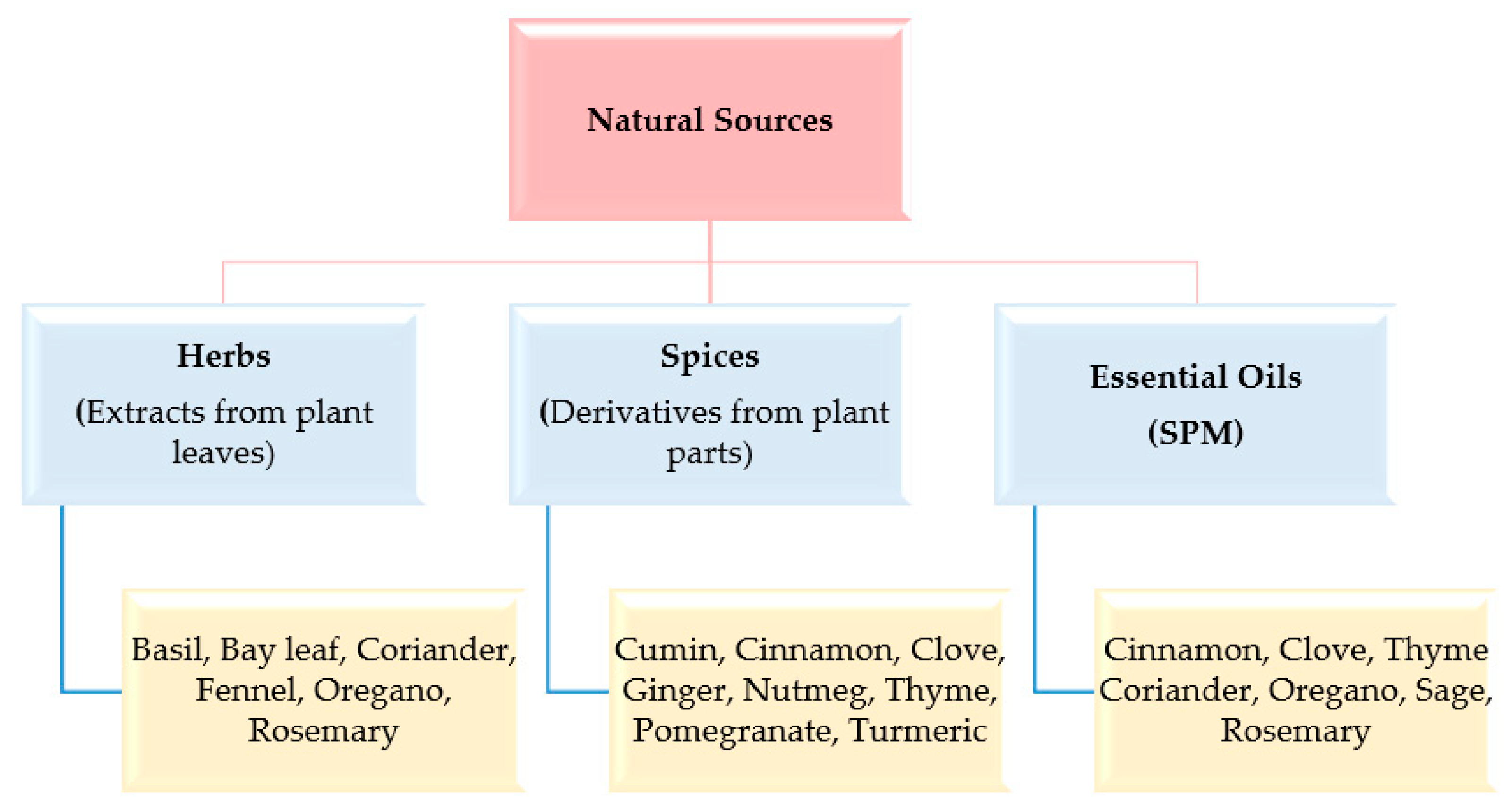
Figure 2. Natural products derived from plants.
Plant phenols are divided into different classes, including simple phenols, phenolic acids, anthocyanins, stilbenes, flavonoids, tannins, lignans, and lignins (Figure 5). Phenolic acids are aromatic secondary plant metabolites that are extensively spread across the plant kingdom. [46]. Currently, there is much scientific interest in their potential protective role against oxidative stress-related diseases. The main edible sources of phenolic acids are fruits, vegetables, cereals, seeds, berries, beverages, olives, and aromatic plants, and they can be found in almost all parts of the plant [47][48]. They occur in the form of esters, glycosides, and amides but rarely in the free form [49]. Despite their origin, these versatile molecules have been reported to possess a wider canvas of biological activities such as antioxidant, antibacterial, antifungal, antiviral, anticancer, anti-inflammatory, anti-diabetic, insecticidal, estrogenic, and keratolytic activities, and many more [50][51][52]. Phenolic compounds are well known for their antioxidant potential and have been extensively used as bio-preservatives to elongate their shelf life, besides their other well-established health benefits. Phenolic acids are non-flavonoid polyphenols that contain a carboxyl group with one or more hydroxyl groups linked to a benzene ring [53][54]. Phenolic acids are known to possess two distinctive carbon frameworks: hydroxybenzoic acid (benzoic acid derivatives) and hydroxycinnamic acid (cinnamic acid derivatives). In these two carbon frameworks, even though the fundamental frame remains the same, the structural variation in the numbers and orientation of hydroxyl groups on the aromatic ring results in a variety of potential derivatives. Hydroxybenzoic acids with seven carbon atoms have a general structure of C6-C1 derived directly from benzoic acid and are present in foods in glucoside form. Some common hydroxybenzoic acids are protocatechuic acid, vanillic acid, syringic acid, and gallic acid. Hydroxycinnamic acids are natural phenylpropanoids having a general structure of C6-C3 derived directly from cinnamic acid and are mostly present in the bound form. Among hydroxycinnamic acids, sinapic acid, caffeic acid, ferulic acid, and p-coumaric acid are the most abundant compounds in foods [55][56] (Figure 3). In both these derivatives, the variation lies in their hydroxylation and methylation patterns in aromatic rings.
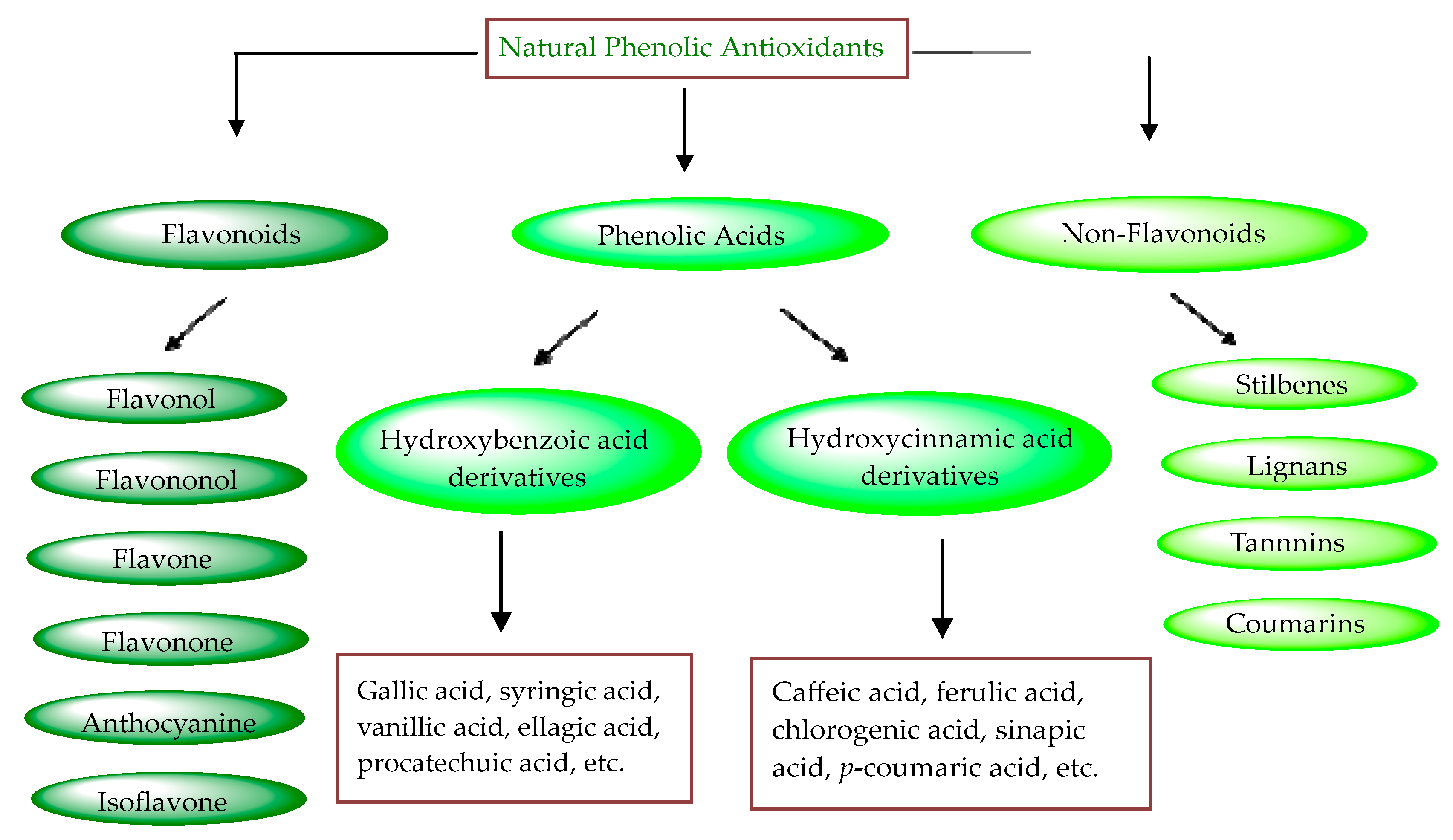
Figure 3. Natural phenolic antioxidants.
Structure–activity relationship (SAR) studies of phenolic acids reveal that the reactivity of the phenol moiety imparts antioxidant activity to a greater extent but can also be affected by some other parameters such as the chemical structure, number, position of hydroxyl groups, substituents on the phenolic ring, and esterification of the carboxyl group. They act as reducing agents, hydrogen donors, and oxygen suppressants when added to food products (Figure 4). They also can inhibit the enzymes involved in radical generation and act as free radical scavengers [57][58]. In general, they are used to prolong the shelf life by preventing oxidative rancidity, degradation, discoloration, contamination, and any other undesirable changes from occurring. Because of their presence in natural plant-based foods and their role as dietary antioxidants, along with radical scavenging abilities, they have received a lot of attention from researchers worldwide [46][59].
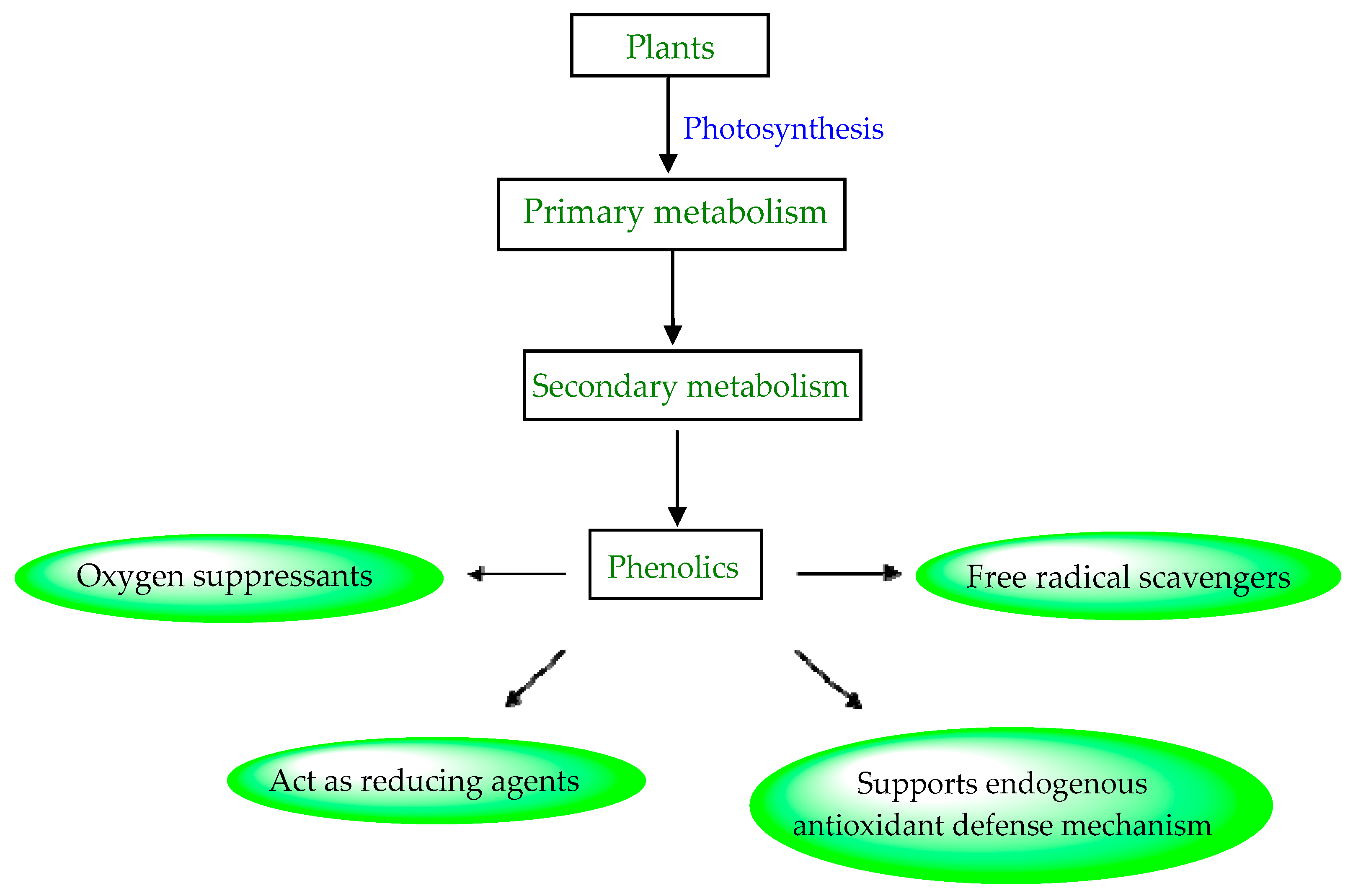
Figure 4. Biosynthesis of phenolic compounds with excellent antioxidant activity.
4.1. Natural Plant Constituents as Antimicrobial/Antioxidants
Antimicrobial agents derived from natural sources have been recognized and used anciently in preservation [60]. The importance of natural sources as antimicrobials/antioxidants is well established and this allows them to be selected as potential candidates for novel preservatives (Table 2 and Figure 5).
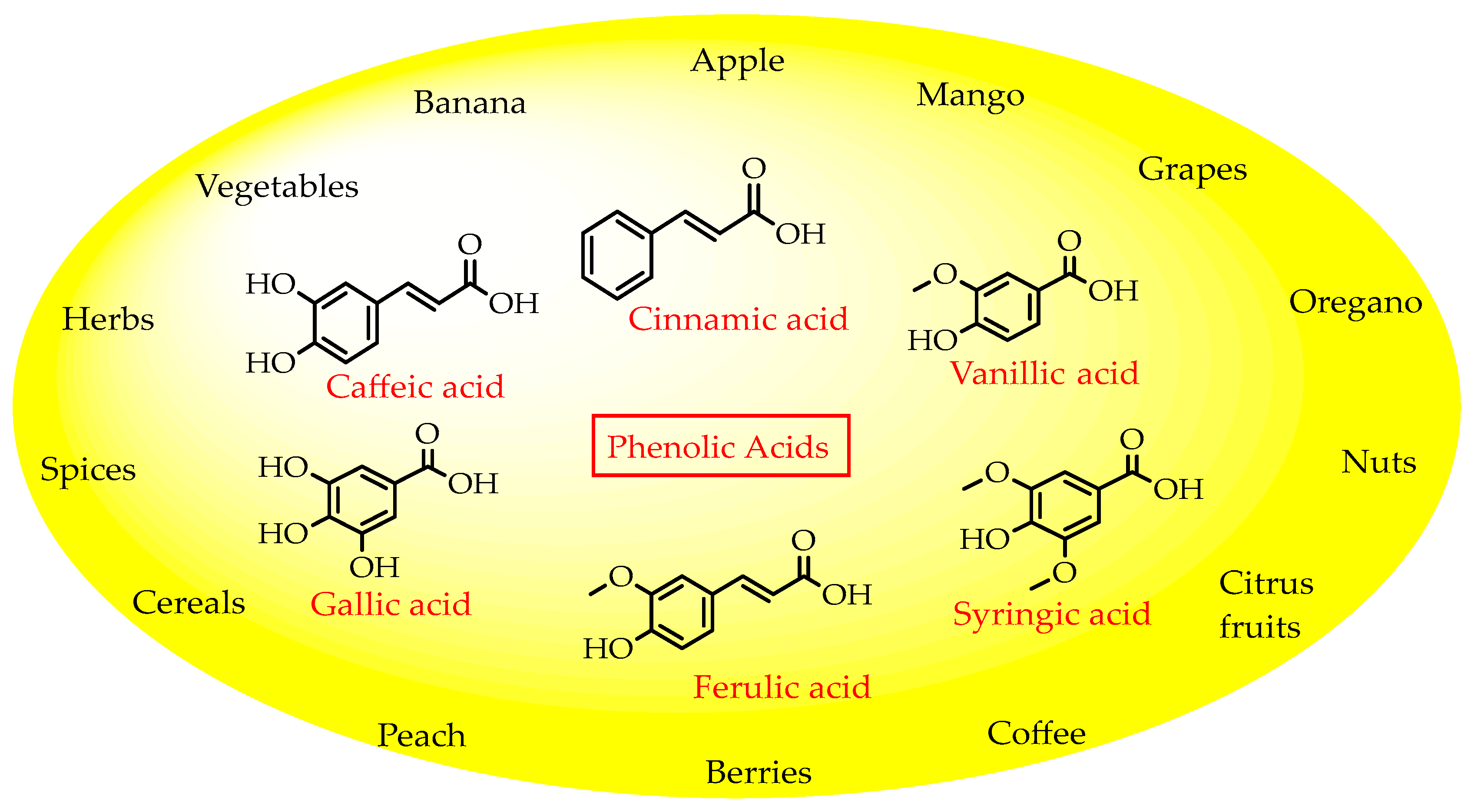
Figure 5. Phenolic acids and their natural sources.
Table 2. List of some phenolic acids and their reported biological activities.
| Natural Sources | Phenolic Content with Antioxidant/Antimicrobial Activity | References |
|---|---|---|
| Allium cepa | Protocatechuic acid | [61] |
| Ananas comosus | Coumaric acid and ferulic acid | [62] |
| Berries | Hydroxycinnamic and hydroxybenzoic acids | [63][64][65] |
| Coffea arabica | Hydroxycinammic acids | [64] |
| Daucus carota | p-Coumaric, chlorogenic, and caffeic acids | [66][67] |
| Emblica officinalis | 1,8-Cineole | [68][69] |
| Fagopyrum esculentum | Rutin, quercetin, and catechins | [70][71][72] |
| Glycine max | Protocatechuic acid, p-hydroxybenzoic acids, chlorogenic acid, etc. | [73] |
| Malus domestica | Coumaric acid, catechin, epicatechin, chlorogenic acid, procyanidin and gallic acid, etc. | [74] |
| Mangifera indica | Myricetin, chlorogenic acid, kaempferol, gallic acid, sinapic acid, and ferulic acid | [75] |
| Mushroom species | p-Hydroxybenzoic, protocatechuic, gallic, vanillic, coumaric, syringic, gentisic, ferulic, cinnamic, caffeic acids | [76] |
| Origanum vulgare | Rosmarinic acid | [77] |
| Oryza sativa | Protocatechuic acid | [78] |
| Psidium guajava | Chlorogenic Acid, gallic acid, Kaempferol, sinapic acid, and myricetin | [75] |
| Punica granatum | Quercetin, kaempferol, luteolin, and Myricetin, etc. |
[79] |
| Salvia rosmarinus | Rosmarinic acid, carnosol, and carnosic acid | [77] |
| Syzygium cumini | Quercetin | [80][81][82] |
| Thymus vulgaris | Caffeic and rosmarinic acids | [83] |
| Triticum aestivum | Phytic acid, ferulic acid, sinapic, syringic, vanillic and p-coumaric acids | [84][85] |
| Vitis vinifera | Malvidin, cyanidin, delphinidin, peonidin and petunidin, epigallocatechin etc. | [86][87] |
4.2. Applications of Phenolic Antioxidant/Antimicrobials
Over the last years, naturally occurring fruits, vegetables, herbs, spices, oils, and their extracts attract the attention of researchers as they are regarded as the ultimate sources of phenolic compounds. They have been tremendously utilized/applied by the food, cosmetics, and pharma industries for a better therapeutic efficacy and conservation of substances. Moreover, they are broadly available, reliable, and cheap with minimal toxic effects, which makes them superior to synthetic products in all aspects. Various applications of polyphenolic compounds [88][89][90][91][92][93] in the pharmaceutical, food, and cosmetics sectors have been summarized in Figure 3.
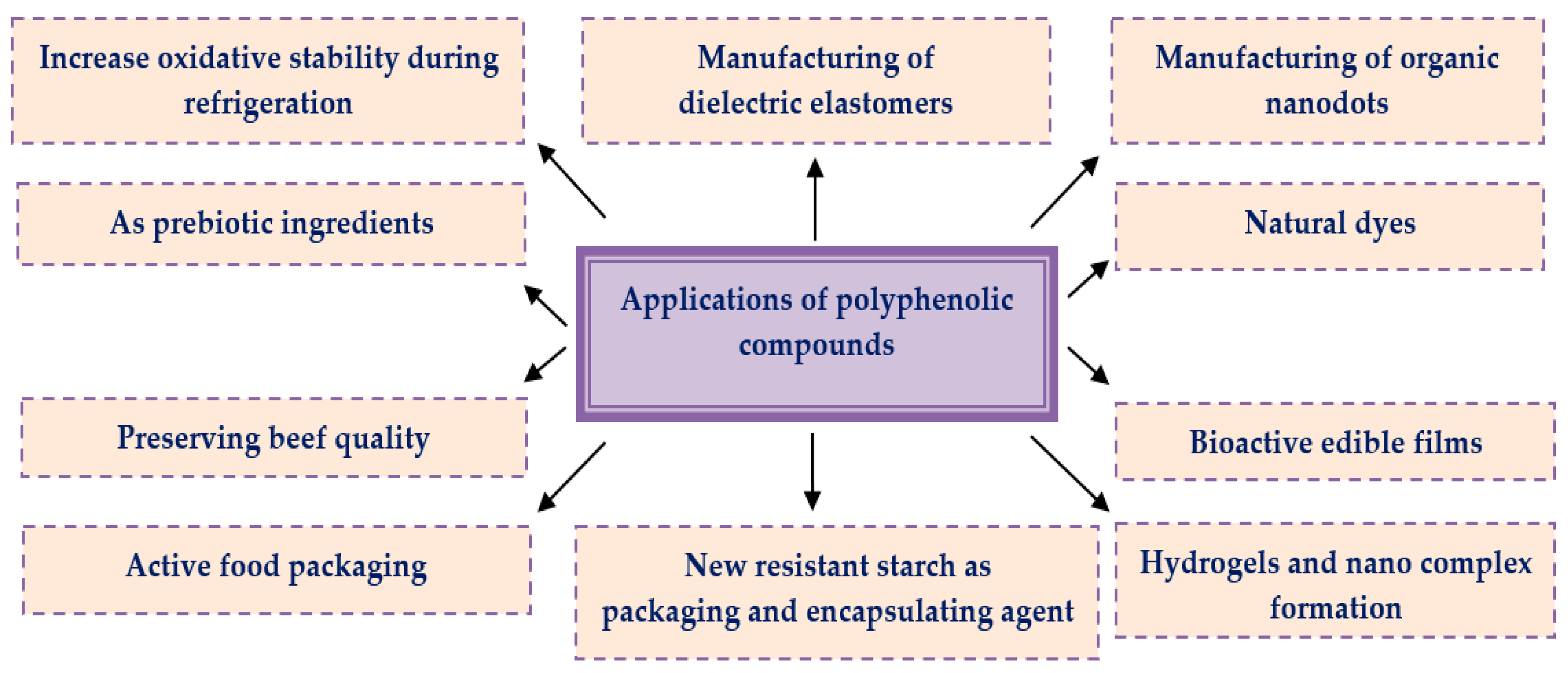
Figure 3. Applications of polyphenolic compounds in different sectors.
This entry is adapted from the peer-reviewed paper 10.3390/ma16134793
References
- Carstensen, J.T.; Rhodes, C. Drug stability, revised, and expanded: Principles and practices. Inf. Health Care 2000, 213, 223.
- Khatkar, A.; Nanda, A.; Narasimhan, B. Preservatives-associated problems and possible alternatives. Curr. Trends Biotechnol. 2012, 2012, 100–120.
- Anand, S.P.; Sati, N. Artificial preservatives and their harmful effects: Looking toward nature for safer alternatives. Int. J. Pharm. Sci. Res. 2013, 4, 2496.
- Yoshioka, S.; Stella, V.J. Stability of Drugs and Dosage Form. Adv. Drug Deliv. Rev. 2002, 54, 273–288.
- Aulton, M.E.; Taylor, K.M. The Design and Manufacture of Medicines; Elsevier Health Sciences: Churchill Livingstone: Edinburgh, Scotland, 2013.
- Singh, Y. Martin’s Physical Pharmacy and Pharmaceutical Sciences; Department of Pharmaceutics Ernest Mario School of Pharmacy Rutgers, The State University of New Jersey: New Brunswick, NJ, USA, 2006.
- Attwood, D.; Rolland, I.P. Chemical kinetics and drug stability. Mod. Pharm. 2009, 1, 221–270.
- Gokoglu, N. Novel natural food preservatives and applications in seafood preservation: A review. J. Sci. Food Agric. 2019, 99, 2068–2077.
- Pezeshk, S.; Seyed, M.O.; Alireza, A. Effect of plant antioxidant and antimicrobial compounds on the shelf-life of seafood-a review. Czech J. Food Sci. 2015, 33, 195–203.
- Zani, F.; Minutello, A.; Maggi, L.; Mazza, P. Evaluation of preservative effectiveness in pharmaceutical products: The use of a wild strain of Pseudomonas cepacian. J. Appl. Microbiol. 1997, 83, 322–326.
- Khatkar, A.; Arun, N.; Balasubramanian, N. Evaluation of preservative effectiveness of ferulic acid derivatives in Aluminium Hydroxide Gel-USP. Int. J. Pharma. Sci. Res. 2013, 4, 2721–2726.
- Gyawali, R.; Salam, A. Natural products as antimicrobial agents. Food Control 2014, 46, 412–429.
- Sharma, S. Food preservatives and their harmful effects. Int. J. Sci. Res. Publ. 2015, 5, 1–2.
- Baig, S.K.M.S.; Kasim, S.S. Study of Harmful Effects of Consuming Food Additives and Public Awareness. IJSRST 2018, 2, 1071–1074.
- WHO. Food Irradiation: A Technique for Preserving and Improving the Safety of Food; World Health Organization: Geneva, Switzerland, 1988.
- Kovanda, L.; Zhang, W.; Wei, X.; Luo, J.; Wu, X.; Atwill, E.R.; Vaessen, S.; Li, X.; Liu, Y. In vitro antimicrobial activities of organic acids and their derivatives on several species of gram-negative and gram-positive bacteria. Molecules 2019, 24, 3770.
- Gómez-García, M.; Sol, C.; de Nova, P.J.G.; Puyalto, M.; Mesas, L.; Puente, H.; Mencía-Ares, O.; Miranda, R.; Argüello, H.; Rubio, P.; et al. Antimicrobial activity of a selection of organic acids, their salts and essential oils against swine enteropathogenic bacteria. Porc. Health Manag. 2019, 5, 32.
- Zapaśnik, A.; Sokołowska, B.; Bryła, M. Role of Lactic Acid Bacteria in Food Preservation and Safety. Foods 2022, 11, 1283.
- Hassan, R.A.; Sand, M.I.; El-Kadi Sh, M. Effect of some organic acids on fungal growth and their toxins production. J. Agric. Chem. Biotechnol. 2012, 3, 391–397.
- Trček, J.; Mira, N.P.; Jarboe, L.R. Adaptation and tolerance of bacteria against acetic acid. Appl. Microbiol. Biotechnol. 2015, 99, 6215–6229.
- Levine, A.S.; Fellers, C.R. Action of acetic acid on food spoilage microorganisms. J. Bacteriol. 1940, 39, 499–515.
- Sheraz, M.A.; Khan, M.F.; Ahmed, S.; Kazi, S.H.; Ahmad, I. Stability and stabilization of ascorbic acid. Househ. Pers. Care Today 2015, 10, 22–25.
- Tfouni, S.A.V.; Toledo, M.C.F. Determination of benzoic and sorbic acids in Brazilian food. Food Control. 2002, 13, 117–123.
- World Health Organization. Benzoic Acid and Sodium Benzoate; World Health Organization: Geneva, Switzerland, 2000.
- Anyasi, T.A.; Jideani, A.I.O.; Edokpayi, J.N.; Anokwuru, C.P. Application of Organic Acids. In Food Preservation, Organic Acids, Characteristics, Properties and Synthesis; Vargas, C., Ed.; Nova Publishers: New York, NY, USA, 2017; pp. 1–47.
- Silva, M.M.; Lidon, F. Food preservatives–An overview on applications and side effects. Emir. J. Food Agric. 2016, 28, 366–373.
- Marz, U. World Markets for Citric, Ascorbic and Isoascorbic Acids: Highlighting Antioxidants in Food; Business Communications Company: Ridgeland, MS, USA, 2002.
- Hietala, J.; Vuori, A.; Johnsson, P.; Pollari, I.; Reutemann, W.; Kieczka, H. Formic acid. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH Verlag GmbH & Co: Hoboken, NJ, USA, 2016; Volume 1, pp. 1–22.
- Alakomi, H.L.; Skytta, E.; Saarela, M.; Mattila-Sandholm, T.; Latva-Kala, K.; Helander, I.M. Lactic acid permeabilizes gram-negative bacteria by disrupting the outer membrane. Appl. Environ. Microbiol. 2000, 66, 2001–2005.
- Shamloo, E.; Hosseini, H.; Abdi Moghadam, Z.; Halberg Larsen, M.; Haslberger, A.; Alebouyeh, M. Importance of Listeria monocytogenes in food safety: A review of its prevalence, detection, and antibiotic resistance. Iran. J. Vet. Res. 2019, 20, 241.
- Mcclure, N.B. The Effect of Lactate on Nitrite in a Cured Meat System. Ph.D. Thesis, Lowa State University, Ames, IA, USA, 2009.
- USDA. Technical Evaluation Report Compiled by OMRI for the USDA National Organic Program, Lactic Acid, Sodium Lactate, and Potassium Lactate, Handling/Processing; Technical Evaluation Report; USDA: Washington, DC, USA, 17 February 2015.
- Adler, G.K.; Hornik, E.S.; Murray, G.; Bhandari, S. Acute effects of the food preservative propionic acid on glucose metabolism in humans. BMJ Open Diabet. Res. Care 2021, 9, e002336.
- European Parliament, Council of the European Union. Directive 2008/7/CE of the European Parliament and of the Council of 10 March 2008; European Parliament: Strasbourg, France, 2008.
- Naveed, N. The perils of cosmetics. J. Pharm. Sci. Res. 2014, 6, 338.
- Lee, J.D.; Lee, J.Y.; Kwack, S.J.; Shin, C.Y.; Jang, H.J.; Kim, H.Y.; Kim, M.K.; Seo, D.W.; Lee, B.M.; Kim, K.B. Risk assessment of triclosan, a cosmetic preservative. Toxicol. Res. 2019, 35, 137–154.
- Dhillon, G.S.; Kaur, S.; Pulicharla, R.; Brar, S.K.; Cledón, M.; Verma, M.; Surampalli, R.Y. Triclosan: Current status, occurrence, environmental risks and bioaccumulation potential. Int. J. Environ. Res. Public Health 2015, 12, 5657–5684.
- Younes, M. EFSA ANS Panel (EFSA Panel on Food Additives and Nutrient Sources Added to Food); Wiley: Hoboken, NJ, USA, 2017; pp. 520–523.
- Lien, E.L. Toxicology and safety of DHA. Prostaglandins Leukot. Essent. Fat. Acids 2009, 81, 125–132.
- Carocho, M.; Morales, P.; Ferreira, I.C. Antioxidants: Reviewing the chemistry, food applications, legislation and role as preservatives. Trends Food Sci. Technol. 2018, 71, 107–120.
- EFSA. EFSA Panel on Food additives and Nutrient Sources added to Food (ANS). Scientific opinion on the re-evaluation of dimethyl dicarbonate (DMDC, E 242) as a food additive. EFSA J. 2015, 13, 4319.
- Yu, Y.; Wu, J.; Xu, Y.; Xiao, G.; Zou, B. Effect of high pressure homogenization and dimethyl dicarbonate (DMDC) on microbial and physicochemical qualities of mulberry juice. J. Food Sci. 2016, 81, 702–708.
- Costa, A.; Barata, A.; Malfeito-Ferreira, M.; Loureiro, V. Evaluation of the inhibitory effect of dimethyl dicarbonate (DMDC) against wine microorganisms. Food Microbiol. 2008, 25, 422–427.
- Anupama, S.; Sharma, P.K.; Garima, G. Natural products as preservatives. Int. J. Pharma Bio Sci. 2010, 1, 612.
- Sharif, Z.I.M.; Mustapha, F.A.; Jai, J.; Yusof, N.; Zaki, N.A.M. Review on methods for preservation and natural preservatives for extending the food longevity. Chem. Eng. Res. Bull. 2017, 19, 145–153.
- Robbins, R.J. Phenolic acids in foods: An overview of analytical methodology. J. Agric. Food Chem. 2003, 51, 2866–2887.
- Ignat, I.; Volf, I.; Popa, V.I. A critical review of methods for characterisation of polyphenolic compounds in fruits and vegetables. Food Chem. 2011, 126, 1821–1835.
- Teodoro, G.R.; Ellepola, K.; Seneviratne, C.J.; Koga-Ito, C.Y. Potential use of phenolic acids as anti-Candida agents: A review. Front. Microbiol. 2015, 6, 1420.
- Pereira, D.M.; Valentão, P.; Pereira, J.A.; Andrade, P.B. Phenolics: From chemistry to biology. Molecules 2009, 14, 2202–2211.
- Chong, K.P.; Rossall, S.; Atong, M. In vitro antimicrobial activity and fungitoxicity of syringic acid, caffeic acid and 4-hydroxybenzoic acid against Ganoderma boninense. J. Agric. Sci. 2009, 1, 15.
- Croft, K.D. The chemistry and biological effects of flavonoids and phenolic acids. Ann. N. Y. Acad. Sci. 1998, 854, 435–442.
- Yang, C.S.; Landau, J.M.; Huang, M.T.; Newmark, H.L. Inhibition of carcinogenesis by dietary polyphenolic compounds. Ann. Rev. Nutr. 2001, 21, 381.
- Khadem, S.; Marles, R.J. Monocyclic phenolic acids; hydroxy-and polyhydroxybenzoic acids: Occurrence and recent bioactivity studies. Molecules 2010, 15, 7985–8005.
- Lafay, S.; Gil-Izquierdo, A. Bioavailability of phenolic acids. Phytochem. Rev. 2008, 7, 301–311.
- Clifford, M.N. Chlorogenic acids and other cinnamates–nature, occurrence and dietary burden. J. Sci. Food Agric. 1999, 79, 362–372.
- Tsao, R.; Deng, Z. Separation procedures for naturally occurring antioxidant phytochemicals. J. Chromatogr. B 2004, 812, 85–99.
- Embuscado, M.E. Spices and herbs: Natural sources of antioxidants—A mini review. J. Funct. Foods 2015, 18, 811–819.
- Campêlo, M.C.S.; Medeiros, J.M.S.; Silva, J.B.A. Natural products in food preservation. Int. Food Res. J. 2019, 26, 41–46.
- Rice-Evans, C.A.; Miller, N.J.; Paganga, G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Rad. Biol. Med. 1996, 20, 933–956.
- Hobson, C.; Chan, A.N.; Wright, G.D. The antibiotic resistome: A guide for the discovery of natural products as antimicrobial agents. Chem. Rev. 2021, 121, 3464–3494.
- Link, K.P.; Angell, H.R.; Walker, J.C. The isolation of protocatechuic acid from pigmented onion scales and its significance in relation to disease resistance in onions. J. Biol. Chem. 1929, 81, 369–375.
- Brito, T.B.N.; Lima, L.R.S.; Santos, M.C.B.; Moreira, R.F.A.; Cameron, L.C.; Fai, A.E.C.; Ferreira, M.S.L. Antimicrobial, antioxidant, volatile and phenolic profiles of cabbage-stalk and pineapple-crown flour revealed by GC-MS and UPLC-MSE. Food Chem. 2021, 339, 127882.
- Jiang, Y.; Fang, Z.; Leonard, W.; Zhang, P. Phenolic compounds in Lycium berry: Composition, health benefits and industrial applications. J. Funct. Foods 2021, 77, 104340.
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747.
- Puupponen-Pimiä, R.; Nohynek, L.; Meier, C.; Kähkönen, M.; Heinonen, M.; Hopia, A.; Oksman-Caldentey, K.M. Antimicrobial properties of phenolic compounds from berries. J. Appl. Microbiol. 2001, 90, 494–507.
- Alasalvar, C.; Grigor, J.M.; Zhang, D.; Quantick, P.C.; Shahidi, F. Comparison of volatiles, phenolics, sugars, antioxidant vitamins, and sensory quality of different colored carrot varieties. J. Agric. Food Chem. 2001, 49, 1410–1416.
- Potter, A.S.; Foroudi, S.; Stamatikos, A.; Patil, B.S.; Deyhim, F. Drinking carrot juice increases total antioxidant status and decreases lipid peroxidation in adults. Nutr. J. 2011, 10, 96.
- Gachkar, L.; Yadegari, D.; Rezaei, M.B.; Taghizadeh, M.; Astaneh, S.A.; Rasooli, I. Chemical and biological characteristics of Cuminum cyminum and Rosmarinus officinalis essential oils. Food Chem. 2007, 102, 898–904.
- Mayachiew, P.; Devahastin, S. Antimicrobial and antioxidant activities of Indian gooseberry and galangal extracts. LWT-Food Sci. Technol. 2008, 41, 1153–1159.
- Inglett, G.E.; Rose, D.J.; Chen, D.; Stevenson, D.G.; Biswas, A. Phenolic content and antioxidant activity of extracts from whole buckwheat (Fagopyrum esculentum Möench) with or without microwave irradiation. Food Chem. 2010, 119, 1216–1219.
- Morishita, T.; Yamaguchi, H.; Degi, K. The contribution of polyphenols to antioxidative activity in common buckwheat and tartary buckwheat grain. Plant Prod. Sci. 2007, 10, 99–104.
- Deleu, C.Q.; Gressier, B.; Vasseur, J.; Dine, T.; Brunet, C.; Luyckx, M.; Cazin, M.; Cazin, J.C.; Bailleul, F.; Trotin, F. Phenolic compounds and antioxidant activities of buckwheat (Fagopyrum esculentum Moench) hulls and flour. J. Ethnopharmacol. 2000, 72, 35–42.
- Taie, H.A.A.; El-Mergawi, R.; Radwan, S. Isoflavonoids, flavonoids, phenolic acids profiles and antioxidant activity of soybean seeds as affected by organic and bioorganic fertilization. Am. Eurasian J. Agric. Environ. Sci. 2008, 4, 207–213.
- Boyer, J.; Liu, R.H. Apple phytochemicals and their health benefits. Nutr. J. 2004, 3, 5.
- Abdullah, N.; Zulkifli, K.S.; Abdullah, A.; Aziman, N.; Saidatul, S.W.K.W. Assessment on the antioxidant and antibacterial activities of selected fruit peels. Int. J. Chem. Tech. Res. 2012, 4, 1534–1542.
- Heleno, S.A.; Martins, A.; Queiroz, M.J.R.P.; Ferreira, I.C.F.R. Bioactivity of phenolic acids: Metabolites versus parent compounds: A review. Food Chem. 2015, 173, 501–513.
- Pokorny, J.; Yanishlieva, N.; Gordon, M.H. Antioxidants in Food: Practical Applications; CRC Press: Boca Raton, FL, USA, 2001.
- Hudson, E.A.; Dinh, P.A.; Kokubun, T.; Simmonds, M.S.J.; Gescher, A. Characterization of potentially chemopreventive phenols in extracts of brown rice that inhibit the growth of human breast and colon cancer cells. Cancer Epidemiol. Biomark. Prevent. 2000, 9, 1163–1170.
- Parashar, A.; Badal, S. Pomegranate juice is potentially better than orange juice in improving antioxidant function in elderly subjects. Elixir Food Sci. J. 2011, 32, 2068–2074.
- Khanduja, K.L.; Gandhi, R.K.; Pathania, V.; Syal, N. Prevention of N-nitrosodiethylamine-induced lung tumorigenesis by ellagic acid and quercetin in mice. Food Chem. Toxicol. 1999, 37, 313–318.
- Swami, S.B.; Thakor, N.S.J.; Patil, M.M.; Haldankar, P.M. Jamun (Syzygium cumini (L.)): A review of its food and medicinal uses. Food Nutr. Sci. 2012, 3, 1100–1117.
- De, S.; Chakraborty, J.; Chakraborty, R.N.; Das, S. Chemopreventive activity of quercetin during carcinogenesis in cervix uteri in mice. Phytother. Res. 2000, 14, 347–351.
- Wu, G.; Chang, C.; Hong, C.; Zhang, H.; Huang, J.; Jin, Q.; Wang, X. Phenolic compounds as stabilizers of oils and antioxidative mechanisms under frying conditions: A comprehensive review. Trends Food Sci. Technol. 2019, 92, 33–45.
- Canan, C.; Delaroza, F.; Casagrande, R.; Baracat, M.M.; Shimokomaki, M.; Ida, E.I. Antioxidant capacity of phytic acid purified from rice bran. Acta Sci. Technol. 2012, 34, 457–463.
- Vaher, M.; Matso, K.; Levandi, T.; Helmja, K.; Kaljurand, M. Phenolic compounds and the antioxidant activity of the bran, flour and whole grain of different wheat varieties. Procedia Chem. 2010, 2, 76–82.
- Yang, J.; Martinson, T.E.; Liu, R.H. Phytochemical profiles and antioxidant activities of wine grapes. Food Chem. 2009, 116, 332–339.
- Xia, E.Q.; Deng, G.F.; Guo, Y.J.; Li, H.B. Biological activities of polyphenols from grapes. Int. J. Mol. Sci. 2010, 11, 622–646.
- Martillanes, S.; Rocha-Pimienta, J.; Cabrera-Bañegil, M.; Martín-Vertedor, D.; Delgado-Adámez, J. Application of Phenolic Compounds for Food Preservation: Food Additive and Active Packaging, Phenolic Compounds–Biological Activity; IntechOpen: London, UK, 2017; pp. 39–58.
- Phan, K.; Van Den Broeck, E.; Van Speybroeck, V.; De Clerck, K.; Raes, K.; De Meester, S. The potential of anthocyanins from blueberries as a natural dye for cotton: A combined experimental and theoretical study. Dye. Pigment. 2020, 176, 108180.
- PJ Garrido, E.M.; Cerqueira, A.S.; Chavarria, D.; Silva, T.; Borges, F.; MPJ Garrido, J. Microencapsulation of caffeic acid phenethyl ester and caffeic acid phenethyl amide by inclusion in hydroxypropyl-β-cyclodextrin. Food Chem. 2018, 254, 260–265.
- Ge, J.; Yue, P.; Chi, J.; Liang, J.; Gao, X. Formation and stability of anthocyanins-loaded nanocomplexes prepared with chitosan hydrochloride and carboxymethyl chitosan. Food Hydrocoll. 2018, 74, 23–31.
- Liu, J.; Wang, X.; Yong, H.; Kan, J.; Jin, C. Recent advances in flavonoid-grafted polysaccharides: Synthesis, structural characterization, bioactivities and potential applications. Int. J. Biol. Macromol. 2018, 116, 1011–1025.
- de Araújo, F.F.; de Paulo Farias, D.; Neri-Numa, I.A.; Pastore, G.M. Polyphenols and their applications: An approach in food chemistry and innovation potential. Food Chem. 2021, 338, 127535.
This entry is offline, you can click here to edit this entry!
