Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Pharmacology & Pharmacy
Psoriasis is an incurable skin disease that develops in about two-thirds of patients before the age of 40 and requires lifelong treatment; its pathological mechanisms have not been fully elucidated. The core pathological process of psoriasis is epidermal thickening caused by the excessive proliferation of epidermal keratinocytes, which is similar to the key feature of cancer; the malignant proliferation of cancer cells causes tumor enlargement, suggesting that there is a certain degree of commonality between psoriasis and cancer.
- psoriasis
- cancer
- cell proliferation
1. Introduction
Psoriasis is one of the most common chronic skin diseases, with clinical features of skin lesions that have raised, well-demarcated, erythematous oval plaques with adherent silvery scales [1]. In addition to skin lesion-related symptoms, psoriatic patients suffer from comorbidities, such as psoriatic arthritis, metabolic syndrome, psychological disorders, cardiovascular disease, atherosclerosis, inflammatory bowel disease, chronic obstructive pulmonary disease, etc. [2]. Psoriasis predominantly develops in young adults, with about two-thirds of patients developing the disease before the age of 40 years, about one-third of patients developing the disease in childhood (age < 18 years), and about 57% of patients suffering from moderate to severe psoriasis [3,4]. In brief, psoriasis is a disease that seriously endangers human health.
Psoriasis is not yet curable and requires lifelong treatment. The choice of treatment for psoriasis depends on several factors, including location, the severity of the disease, and whether it is accompanied by other diseases [5]. Topical therapy involving corticosteroids, vitamin D analogues, tapinarof, and calcineurin inhibitors is the gold standard for patients with mild psoriasis [6]. Oral systemic therapies are the conventional treatment options for patients with moderate to severe psoriasis, with oral systemic agents such as methotrexate, acitretin, ciclosporin, dimethyl fumarate, apremilast, and tofacitinib [7]. Biological therapies are increasingly used to treat moderate to severe, refractory, and special types of psoriasis [8]. Currently, biologics targeting TNF-α, IL-12/IL-23 p40, IL-23p19, IL-17A, and IL-36R are used in the clinical treatment of psoriasis, and they are superior in terms of efficacy than nonbiological treatment [9,10]. Phototherapy can be safely combined with other therapeutic options to improve the effects of therapy [11]. Oral small-molecule targeted drugs are promising options for treating psoriasis, providing advantages over biologics in terms of patient convenience, reduced healthcare costs, and improved quality of life [12]. Currently, the available oral small-molecule targeted drugs for treating psoriasis include the phosphodiesterase 4 (PDE4) inhibitor apremilast and the tyrosine-protein kinase 2 (TYK2) inhibitor deucravacitinib [12]. In summary, a range of treatment methods for psoriasis has been developed over the past few decades. Despite the existence of many therapeutical options, psoriasis still cannot be completely cured owing to treatment failure and disease relapse. Therefore, there is an urgent need to investigate the molecular and genetic mechanisms of psoriasis pathogenesis from different perspectives in order to develop new therapeutic targets and drugs.
The pathological mechanisms of psoriasis have not been fully elucidated. The key pathological features of psoriasis are excessive keratinocyte proliferation, abnormal differentiation, and the infiltration of a variety of immunoinflammatory cells. Additionally, its accepted pathogenesis is that the interaction between hyperproliferative epidermal keratinocytes and activated immune cells interact to form a positive feedback loop (Figure 1) [5,13]. The pathological changes related to psoriasis are similar to the key feature of cancer—that is, the malignant proliferation of cancer cells causes tumor enlargement, suggesting that there is a certain degree of commonality between psoriasis and cancer [13,14].
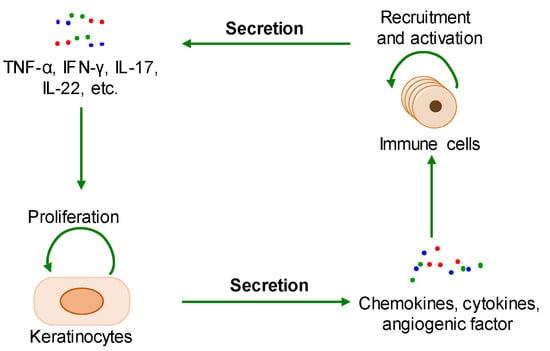
Figure 1. The positive feedback loop of a keratinocyte-immune microenvironment. TNF-α: tumor necrosis factor alpha; INF-γ: interferon-gamma; IL-17: interleukin-17; IL-22: interleukin-22.
2. Common Pathological Mechanisms between Psoriasis and Cancer
A great deal of research has shown that the interaction between cell proliferation and abnormal immune microenvironment, metabolic reprogramming, and epigenetic reprogramming are common pathological mechanisms between psoriasis and cancer, and there are common pathogenic genes or proteins in these shared pathological mechanisms.
2.1. The Interaction between Cell Proliferation and Abnormal Immune Microenvironment
During the onset of psoriasis, external stimuli trigger the recruitment and activation of immune cells to secrete cytokines such as IL-17, IL-22, TNF-α, and INF-γ, which promote keratinocyte proliferation. The proliferative keratinocytes secrete cytokines, chemokines, and angiogenic factors such as TNF-α, IL-8, CXCL8, and VEGF, which promote angiogenesis and the recruitment and activation of immune cells. Thus, the immune microenvironment of psoriatic lesions is characterized by angiogenesis, increasing proinflammatory factors, and immune cells. Additionally, external stimuli also induce keratinocyte proliferation and the secretion of proinflammatory factors [15,16]. In summary, the proliferative keratinocytes and activated immune cells form a positive loop mediated by proinflammatory factors, maintaining and accelerating the process of psoriasis (Figure 2).
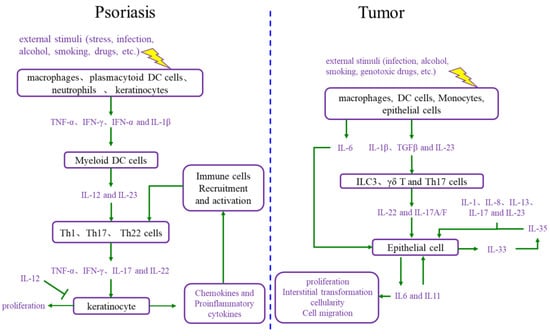
Figure 2. Comparison of the interaction between cell proliferation and immune microenvironment in psoriasis and cancer. DC cells: dendritic cells; IL-12: interleukin-12; IL-23: interleukin-23; Th1 cells: T helper 1 cells; Th17 cells: T helper 17 cells; Th22 cells: T helper 22 cells; TNF-α: tumor necrosis factor alpha; INF-γ: interferon gamma; IL-17: interleukin-17; IL-6: interleukin-6; IL-1β: interleukin-1β; TGFβ: transforming growth factor-β; ILC3 cells: group 3 innate lymphoid cells; γδ T cells: gamma delta T cells; IL-1: interleukin-1; IL-8: interleukin-8; IL-13: interleukin-13; IL-35: interleukin-35; IL-33: interleukin-33; IL-11: interleukin-11.
The immune microenvironment is a hallmark of cancer, and the tumor immune microenvironment is more complex than the psoriatic immune microenvironment [14]. The tumor immune microenvironment includes a set of cell types, such as endothelial cells, pericytes, immune inflammatory cells, and cancer-associated fibroblasts [17]. These cells secrete IL-6, IL-23, IL-22, IL-17, IL-1β, and TGF-β, causing cancer cell proliferation. Moreover, as the foundation of cancer, proliferative cancer cells also produce IL-6, IL-11, and other cytokines, leading to malignant proliferation and producing matrix metalloproteinases (MMPs), VEGF, and other adhesion factors, which promote angiogenesis for the nutrition supplementation of tumor cell proliferation and tumor cell circulation, as well as immune cell recruitment and activation [18,19]. Ultimately, the tumor immune microenvironment and proliferative cancer cells form a positive loop, driving tumor progression forward.
2.2. Metabolic Reprogramming
Metabolic reprogramming is another hallmark of cancer. The metabolic pattern of starved rapidly proliferating tumor cells changes in order to meet nutritional requirements [20]. Tumor cell proliferation mainly depends on glycolysis, referred to as the Warburg effect. To maintain the Warburg effect, the expression of glucose transporter 1 (GLUT1), pyruvate kinase (PK), hexokinase (HK), and phosphoglycerate mutase (PGAM) increases in tumors. Meanwhile, the TCA cycle is reprogrammed by the upregulation and mutation of fumarate hydratase (FH), succinate dehydrogenases (SDHs), and isocitrate dehydrogenase 1/2 (IDH1/2). In addition, the changes in enzymes such as glutamine transporter, glutaminase (GLS1), arginine succinate synthase (ASS1), phosphoglycerate dehydrogenase (PHGDH), phosphoserine aminotransferase 1 (PSAT1), serine hydroxymethyltransferase 1/2 (SHMT1/2), and indoleamine 2, 3-dioxygenase 1/2 (IDO1/2) allows for the metabolic reprogramming of amino acids such as glutamine, arginine, serine, and tryptophan metabolic pathways. Sphingomyelin metabolites are also involved in tumor development [21]. In summary, the glucose, amino acid, and lipid metabolisms are reprogrammed in cancer.
The glucose, amino acid, and lipid metabolisms also undergo metabolic reprogramming in psoriasis patients. Hyperproliferative keratinocytes are subjected to the Warburg effect. GLUT1 is required for glucose uptake by proliferative keratinocytes, and its expression is increased in hyperproliferative keratinocytes [22]. Pyruvate kinase isozyme type M2 (PKM2) is generally increased in psoriasis patients and proliferates keratinocytes to enhance glycolysis [23]. IL-17 downregulates protein phosphatase 6 (PP6) and induces the generation of arginase-1 (ARG1) in psoriasis [24]. The deficiency of PP6 leads to the accumulation of AGR1, promoting skin inflammation by driving polyamine production. The IL-17A/MALT1/c-Jun axis induces the abnormal expression of GLS1 in immune cells and keratinocytes [25]. Sphingolipid metabolite sphingosine-1-phosphate (S1P) is increased in psoriasis patients [26].
2.3. Epigenetic Reprogramming
Epigenetics refers to heritable molecular processes whereby a constant DNA sequence produces variable gene expression patterns without any mutational change in the DNA sequence. Compared to normal cells, tumor cells exhibit epigenetic changes such as DNA methylation, histone modifications, and genome-wide changes in the three-dimensional (3D) chromatin structure representing epigenetic mutations [27,28]. In tumor cells, alterations in the activity of epigenetic-related proteins such as histone deacetylases (HDACs), bromodomain proteins (BRDs), TET protein demethylases, histone lysine methyltransferases (HMTs), and lysine-specific demethylases (LSDs) result in abnormal histone acetylation and methylation; DNA methyltransferases (DNMT) aberrantly affect DNA/RNA methylation [27,28]. These epigenetic reprogrammings cause features such as excessive cell proliferation, death resistance, immune escape, genomic instability, and microbiome polymorphisms.
In psoriasis, epigenetic reprogramming occurs by affecting enzymes such as HADCs, BRDs, and HMTs, resulting in abnormal histone acetylation and methylation, as well as abnormal DNA methylation. It has been reported that global DNA methylation is increased in psoriasis patients, and genes with hypermethylated promoters enrich gene ontology processes, such as cell development and differentiation, actin cytoskeleton organization, cell adhesion, and motility; meanwhile, genes with hypomethylated promoters enrich immune activation processes, including inflammation, T cell activation, cytokine production, and cell proliferation [29,30]. Methylation modifications at CpG sites are increased in psoriatic lesions [31]. The promoter of the p16INK4a gene is methylated in the epidermis of psoriasis patients, and p16INK4a mRNA expression is also elevated, with high degrees of methylation of the promoter region and hypomethylation of some other regions also occurring in CD4+ cells [32]. Enhancer homolog 2 of the Zeste gene (EZH2) is a histone H3K27 methylase, whereas EZH2 acts on kallikrein-8 (KLK8) to cause the abnormal proliferation of keratinocytes in psoriasis [32].
3. Common Therapeutic Agents and Therapeutic Targets in Psoriasis and Cancer
3.1. Current Therapeutic Agents and Their Targets
3.1.1. Cytotoxic Agents Regulating the Cell Cycle
The inhibitors of human topoisomerase I and II are conventional cytotoxic agents for the treatment of cancer and psoriasis [44,45]. Human DNA topoisomerase I is essential for DNA replication and is expressed at much higher levels in proliferative cells. Camptothecin, a topoisomerase I inhibitor, is a first-line anticancer drug that has been developed as a topical drug for psoriasis treatment since the early 1970s in China [46,47]. Human DNA topoisomerase II (Topo II) comprises two subtypes: Topo IIα and Topo IIβ. Topo IIα is a key protein required for cell proliferation and Topo IIβ is not related to cell proliferation [48]. Two antitumor Topo II inhibitors, bimolane and ICRF-154, were used in the oral treatment of psoriasis from 1973 to 2006 in China [49,50]. The approved Topo II inhibitors cannot selectively inhibit Topo IIα, but they can inhibit Topo IIβ, causing DNA damage in normal cells [51,52]. Small molecule inhibitors targeting cyclin-dependent kinases (CDKs) play an important role in the evolving field of anticancer treatment [53]. CDK7 expression was markedly increased in CD4+ T cells from patients with psoriasis, and its inhibitor THZ1 ameliorated psoriasiform symptoms in an imiquimod-induced psoriasis-like mouse model [54]. Paclitaxel is a chemotherapeutic agent targeting microtubules, and micellar paclitaxel has demonstrated therapeutic activity in patients with severe psoriasis [55,56]. Methotrexate is an antitumor drug that mainly inhibits DNA synthesis by inhibiting dihydrofolate reductase, thereby inhibiting the growth of tumor cells [57]. Methotrexate is also a commonly used therapeutic agent in psoriasis; its anti-psoriasis mechanism is complex and includes inhibitory effects on folate-dependent enzyme, NF-κB, lincRNA-p21, NO synthase, and solute-carrier family 46 member 2 (SLC46A2) [58].
3.1.2. Immune Modulators
The JAK/STAT signaling pathway plays a critical role in the signaling of a wide array of cytokines and growth factors, and the dysregulation of the JAK/STAT pathway is associated with various cancers and autoimmune diseases [59]. Currently, at least nine small-molecule inhibitors of the JAK/STAT signaling pathway are in development for moderate to severe psoriasis and psoriatic arthritis [60,61]. Deucravacitinib is a first-in-class TYK2 small molecule inhibitor that received approval in the USA on 9 September 2022 for the oral treatment of adults with moderate to severe plaque psoriasis [62]. The pharmacologic inhibition of TYK2 with deucravacitinib decreased proliferation and induced apoptosis over time in malignant peripheral nerve sheath tumors in a dose-dependent manner [63]. The biological targeted agent tildrakizumab specifically antagonizes IL-23 and targets the p19 subunit of IL-23 to inhibit inflammation; it has been used in the clinical treatment of psoriasis while regulating the tumor microenvironment and has been in phase I/II clinical studies for the treatment of prostate cancer [64,65]. The CJM112 monoclonal antibody targets IL-17 to inhibit inflammation and is in phase I clinical studies for the treatment of psoriasis, multiple myeloma, colon cancer, and breast cancer [66,67,68].
3.1.3. Epigenetic Modulators
JQ1, which targets BRD4 and exhibits therapeutic utility in cancer, inhibited the proliferation of keratinocytes and modulated the RORC/IL-17A pathway in a mouse model of psoriasis-like inflammation [69,70,71,72]. The p300/CBP inhibitor A485 displays selective inhibitory activity against several hematological malignancies and androgen receptor-positive prostate cancer [73]. A485 normalizes psoriatic fibroblast gene expression in vitro and reduces psoriasis-like skin inflammation in vivo [74]. Vorinostat, an anti-tumor epigenetic modulator commonly used in the treatment of hematologic cancer, targets HDACs to inhibit keratinocyte proliferation and is undergoing preclinical studies for the treatment of psoriasis [75,76]. Decitabine, a DNA methyltransferase inhibitor, is used to treat myelodysplastic syndromes and ameliorates the imiquimod-induced mouse model of psoriasis [77,78].
3.1.4. Metabolic Modulators
GLUT1 is identified as a novel therapeutic target for psoriasis, and its topical inhibition with WZB117 decreases psoriasiform hyperplasia by inhibiting keratinocyte proliferation in mouse models of psoriasis-like disease [22]. GLUT1 is also a promising drug target for cancer treatment [79]. WZB117 has been shown to significantly inhibit A549 tumor growth over a period of 10 weeks [80]. PKM2, a valuable therapeutic target for cancer, mediates IL-17 signaling in keratinocytes driving psoriatic skin inflammation [81,82]. 2′-Hydroxycinnamaldehyde (HCA), the active component isolated from the stem bark of Cinnamomum cassia, exerts anticancer effects through multiple mechanisms and ameliorates imiquimod-induced psoriasiform inflammation by targeting PKM2-STAT3 signaling in mice [83,84].
3.1.5. Others
VEGF antagonists target VEGF to inhibit angiogenesis and have been used in the clinical treatment of tumors. They have shown clinical efficacy in the treatment of psoriasis, as evidenced by a case report that VEGF antagonists lead to a significant improvement in the psoriasis of metastatic cancer patients [85,86]. Calcipotriol, a vitamin D receptor agonist, is the topical gold standard for the treatment of psoriasis and is able to reverse cisplatin resistance and enhance the efficacy of PD1 antibodies in the treatment of pancreatic ductal adenocarcinoma in gastric cancer [87]. Hsp90 is a highly abundant and ubiquitous molecular chaperone that plays an essential role in cell proliferation; it is a hot topic in the research and development of anti-tumor drugs. Hsp90 inhibitor RGRN-305 has been found to relieve psoriatic lesions in cancer patients in phase I clinical trials for the treatment of cancer, and it has shown good efficacy and safety in phase Ib clinical trials for the treatment of plaque psoriasis [88,89,90]. T-LAK cell-oriented protein kinase (TOPK) is a marker of tumor progression because it is a potent promoter of the malignant proliferation of tumor cells. The TOPK inhibitor OTS514 induces the cytokinesis defect of cancer cells, suggesting that the inhibition of TOPK may be a viable therapeutic option for the treatment of various cancers [91]. TOPK levels are predominantly upregulated in the epidermal keratinocytes of psoriatic lesions in both psoriasis patients and model mice and are positively associated with psoriasis progression. TOPK was upregulated in psoriatic lesions. The topical application of OTS514 clearly alleviates epidermal hyperplasia by inducing G2/M phase arrest and the apoptosis of keratinocytes [92]. All of this indicates that more and more studies have shown that some drugs exhibit effectiveness in the treatment of psoriasis and tumors.
3.2. Novel Potential Therapeutic Targets
More and more studies have shown that some proteins are becoming novel potential therapeutic targets for psoriasis and tumors. KRT17 is overexpressed in both psoriatic keratinocytes and tumor cells, and the topical application of KRT17 siRNA relieves psoriasiform lesions in mice and exhibits antitumor effects in gastric cancer cells [93,94]. TWEAK is a cytokine, and TWEAK and its receptor Fn14 are upregulated in cancer and promote cancer progression. The TWEAK antibody RG7212 has been in clinical trials for the treatment of cancer [95,96,97]. TWEAK is elevated in the serum and skin lesions of psoriasis patients, and the TWEAK antibody and knockout of Fn14 have shown an alleviating effect on psoriasis in mice [98]. Proprotein convertase subtilisin-kexin type 9 (PCSK9) is a sensitive gene in psoriasis, and its protein is elevated in the skin lesions and serum of psoriasis patients; knockout of PCSK9 slows the development of psoriasis-like skin lesions and inflammation in mice [99,100,101]. PCSK9 expression is elevated in a variety of cancers, and knockout of PCSK9 inhibits tumor cell proliferation, induces apoptosis, inhibits tumor growth in mice, and prolongs survival in tumor-bearing mice [102]. Angiopoietin-like protein 4 (ANGPTL4) is highly expressed in the skin lesions of psoriasis patients, ANGPTL4 recombinant protein promotes psoriasis-like skin lesions in mice, and the knockout of ANGPTL4 inhibits keratinocyte growth and expresses cytokines such as IL-17, IL-6, and TNF-α [103]. ANGPTL4 is highly expressed in pancreatic cancer tumor tissues and is associated with the progression of pancreatic cancer. The overexpression of ANGPTL4 promotes pancreatic cancer tumor growth in mice, and ANGPTL4 knockdown inhibits pancreatic cancer tumor growth in mice [104]. The above studies indicate that KRT17, TWEAK, PCSK9, and ANGPTL4 have the potential to serve as therapeutic targets for psoriasis and tumors and require the development of nucleic acid drugs, biologics, and small-molecule drugs against them.
This entry is adapted from the peer-reviewed paper 10.3390/ijms241814390
This entry is offline, you can click here to edit this entry!
