Lactoferrin (LF) is a component of the whey protein of milk of most mammals, probably with the exception of dogs and rats. The concentration of lactoferrin in milk depends on the phase of lactation. It has been proven that colostrum can contain up to seven times more LF than mature milk [10]. Human body cells can produce lactoferrin and it is also found in many organs and cells of the human body. Its presence has been confirmed in kidneys, lungs, gallbladder, pancreas, intestine, liver, prostate, saliva, tears, sperm, cerebrospinal fluid, urine, bronchial secretions, vaginal discharge, synovial fluid, umbilical cord blood, blood plasma, and cells of the immune system [10,11,12]. It is present wherever the body needs quick and effective protection against external threats.
1. Immune System—Effects of Lactoferrin on Foetus, Infants and Reproduction
When discussing lactoferrin, it is often said that it is “unable” to affect the body due to digestion; however, scientific evidence suggests that it is hydrolyzed to stable, immunologically active peptides upon contact with the acidic environment of the gastric juice. Lactoferrin preparations are effective after oral administration, which has been confirmed in numerous studies, including clinical ones [
32,
34,
35,
36,
37,
38]. LF can reach the intestine, mainly in the form of peptide fragments, where they act locally on the microbiota and the immune system associated with the local mucosa, thereby enhancing the immunity of all mucous membranes in the body [
11,
39,
40].
In children suffering from diarrhea, oral lactoferrin both alleviated the course and reduced the frequency [
40,
41]. Published studies using lactoferrin in children have positively evaluated its use for both gastrointestinal infections and sepsis in neonates, and lactoferrin supply in preterm infants is recommended to be introduced as soon as possible [
42,
43]. It is also very interesting that, in pregnant women, intravaginal lactoferrin is one of the preventive measures reducing the risk of premature delivery [
10,
41]. LF supports normal tissue development in the fetus, including normal ossification, adequate iron availability and absorption, protects against infection and inflammation, and benefits both mother and fetus [
44]. LF acts as a probiotic, protecting the lower genital tract and preventing the consequences of inflammation both during pregnancy and before pregnancy, thus aiding fertility. Lactoferrin is present in the follicular fluid, but results of studies on its effect on oocyte maturation and quality are inconclusive [
45,
46]. The role of lactoferrin in male fertility is still under intense debate and research [
47,
48]. It can protect against infections of the male genital tract and regulate iron levels in sperm, thus influencing its quality. This appears to be a good potential marker of sperm quality [
47]. This issue is the subject of ongoing clinical trials—ClinicalTrials.gov Identifier: NCT05171504.
2. Antitoxic and Antipathogenic Properties
Numerous studies have confirmed the beneficial effects of LF on the intestinal epithelium. This protein stimulates the growth, differentiation and secretory activity of epithelial cells, which optimizes the digestive processes and absorption of nutrients and protects against the action of pathogens and food allergens [
61,
62]. LF also protects the intestinal epithelium from the toxic effects of reactive oxygen species (ROS), bacterial toxins and xenobiotics such as nonsteroidal anti-inflammatory drugs (NSAIDs) [
31,
63,
64,
65]. Importantly, LF also protects against gastrointestinal tract infections, both viral and bacterial, fungal and protozoal [
32,
66]. Many tests have demonstrated the protective effect of LF in the states of endotoxemia, bacteremia, sepsis and necrotic enteritis in neonates [
35,
36,
58,
67,
68,
69], in inflammatory colitis [
70,
71] and after partial bowel resection [
72]. LF has antibacterial properties in relation to Gram-negative and Gram-positive bacteria, thanks to which it is helpful for fighting pathogens, prevents the formation of biofilm by pathogenic bacteria, such as
Staphylococcus aureus or blue oil rod (
Pseudomonas aeruginosa) [
66,
73]. LF supports the treatment of gastric infection caused by
Helicobacter pylori [
74]. The mechanism of action of LF may,
inter alia, include the direct inhibition or killing of microbial cells, activation/inhibition of the immune system, or enhancement of intestinal epithelial tightness by stimulating the production of tight junction proteins. In addition, the binding of iron by LF makes its absence associated with a concomitant halt in bacterial growth, which protects the body from infection. [
20]. It also has an immunomodulating effect, stimulating the body to synthesize cytokines and chemokines as well as accelerating the maturation of cells of the immune system [
17,
50,
51,
75].
Human lactoferrin (abbreviated as hLF,) possesses 77% similarities with the bovine form (bLF) in the aspect of amino acid sequences, although bovine lactoferrin is usually studied, because it is easier to obtain. It has been estimated that in a glass of cow’s milk we will find about 25-75 mg of this protein. At the same time, it seems that bLF is not an ideal choice due to differences that may alter its antiviral and antimicrobial potential when used in human therapy, but some authors highlight its stronger antimicrobial activity [
76].
3. Anticancer Activity
One of the many properties of LF is its anticancer activity. This may be related not only to preventing antioxidant stress and inflammation, which contribute to DNA damage and tumorigenesis, but also to preventing the development of, or inhibiting, cancer by stimulating the adaptive immune response [
7]. This is the case for colorectal cancer, the epidemiology of which is mainly related to age and lifestyle factors [
84] and in the case of childhood leukemia where long-term consumption of breast milk may prevent the risk of developing leukemia due to the immunoprotective properties of the LF present [
85]. Furthermore, LF may directly inhibit proliferation, survival, migration, metastasis and accelerating cancer cell death [
86,
87].
It has been confirmed that, in the presence of LF, various cancer cells undergo remarkable damage such as cell cycle arrest, damage to the cytoskeleton and induction of apoptosis, as well as decreased cell migration [
13,
86]. The postulated property of LF by which it activates signaling pathways to generate deleterious effects on cancer cells may be interaction with proteoglycans, glycosaminoglycans, and sialic acid, high levels of which are presented by cancer cells. This may also explain the high cytotoxic selectivity of LF against cancer cells only [
13,
87,
88]. Besides, the ability of LF to enter the cell nucleus is likely the primary mechanism by which it exerts its pleiotropic functions, including anticancer. Nuclear LF (called delta) acts as a transcription factor and causes activation of expression target genes such as Bax, SelH, DcpS, UBE2E1, Skp1 and GTF2F2 and shows the anticancer, anti-proliferating and pro-apoptotic activities [
89,
90,
91,
92]. This corresponds to decreased levels of LF and delta LF expression in tumor cells, which often correlates with greater tumor progression and poor prognosis [
91,
93,
94].
LF also binds iron, which is heavily involved in the metabolic requirements of some cancer cells, and blocks angiogenesis, i.e., prevents the formation of new blood vessels, thereby inhibiting tumor growth and metastasis or directing the tumor toward apoptosis [
21,
95].
An interesting feature of LF that deserves attention, in addition to its proven safety and its low antigenicity and selectivity for cancer cells, which could be used in brain tumor therapy, is its passage through the blood–brain barrier [
87].
4. Aging and Aging-Related Diseases
Aging can be defined as: “the progressive accumulation of changes with time associated with or responsible for the ever-increasing susceptibility to disease and death which accompanies advancing age”, and the factors that lead to aging: “the sum of the deleterious free radical reactions going on continuously throughout the cells and tissues constitutes the aging process or is a major contributor to it” [
96] and “changes in molecular structure and, hence, function” [
97]. In summary, aging is a complex natural phenomenon occurring as a consequence of the passage of time, environmental factors and genetics that increase susceptibility to developing systemic diseases, including metabolic disorders (diabetes mellitus), cardiovascular, neurodegenerative and respiratory diseases as well as rheumatoid arthritis, cancers or dementia [
8,
98,
99,
100].
The pleiotropic anti-aging effect of LF is related to its antioxidant, anti-inflammatory and anticancer effects, as well as the assurance of neuroprotection or the alleviation of mitochondrial dysfunction and systemic disorders [
8].
LF antioxidant potential leads to cells’ and organs’ protection finally extending its lifespan [
101]. In addition, due to regulation of numerous genes expression (inhibition of NF-κB, mTORC1 and caspase via the Erk and Akt pathways), LF regulates cell growth, proliferation, apoptosis and inflammation. It suppresses the senescence and apoptosis of mesenchymal stem cells (MSCs) [
102,
103], promotes both the formation of granulation tissue and re-epithelialization (proliferation and migration of fibroblasts and keratinocytes stimulation and enhancement of extracellular matrix components synthesis) [
104,
105]. Moreover, due to the induction of the targeted apoptosis of senescent cells or the disruption of the senescence-associated secretory phenotype (SASP), LF restores tissue homeostasis [
99,
100]. Interestingly, LF usefulness has been shown for treatment, diagnosis or monitoring age-related diseases [
106,
107,
108,
109,
110,
111,
112,
113,
114,
115,
116,
117,
118,
119,
120]. For example, it may act as a neuroprotective agent in Alzheimer’s disease (AD) and Parkinson’s disease (PD) [
111,
115,
116] leading to the improvement of cognitive function and attenuation of brain senescence [
121]. The possible mechanism of LF action includes iron-binding dependent manner (upregulation of divalent metal transporter 1 (DMT1) and transferrin receptor (TFR) and downregulation ferroportin 1 (Fpn1)) [
117,
118] and/or iron-binding independent manner (regulation of the p-Akt/PTEN or the ERK-CREB pathway in HIF-1-dependent manner) [
118,
119,
120]. Furthermore, LF preserves mitochondrial calcium homeostasis in degenerated dopaminergic neurons [
122]. Moreover, LF regulates body fat metabolism limiting obesity (probable downregulation of adipogenic genes and upregulation of fatty acid synthase and acetyl CoA carboxylase in adipocytes) [
123,
124] and glucose metabolism in patients with type 2 diabetes mellitus via improvement of the insulin-signaling response in adipocytes (up-phosphorylation of Akt serine 473 and up-expression of glucose transport 4 and insulin receptor 1) [
107,
124,
125,
126].
5. Lactoferrin in the Human Diet and Therapy of Diseases
Lactoferrin is an important component in the human diet. Due to its high nutritional value, its antibacterial, antiviral, anti-cancer properties and regulation of the activity of the immune system [
17], it has also been used in the pharmaceutical and food industries and in the production of feed additives. Currently, we can find it in products such as dietary supplements and infant formula. Lactoferrin obtained from cow’s milk is used, among others, in the production of infant formulas, foodstuffs for special medical purposes, milk, yoghurt drinks, ice cream and cookies, dietary supplements, and processed cereal products. It is also appreciated in the cosmetic (e.g., in cosmetics and toothpaste) and pharmaceutical industries [
42,
44,
130,
131].
It is also worth mentioning that LF, as a naturally occurring protein in saliva and produced by salivary glands in the oral cavity, has protective properties and is supposed to provide homeostasis in the oral cavity [
17]. The ability to bind iron ions by LF provides antibacterial activity in the oral cavity. The use of products with LF further supplements it in the oral or nasal cavity, thus strengthening the first protective barrier against bacteria and viruses from the outside [
60,
139,
140,
141,
142,
143]. The effectiveness of LF has been established in numerous in vitro, animal, and human studies in which LF, used in oral and vaginal formulations, positively altered the ecosystem of the reproductive tract by eliminating pathogenic microorganisms and increasing Lactobacillus species, re-establishing the state of eubiosis and protecting from dangerous consequences of dysbiosis, such as premature labor or miscarriage [
6,
144].
So far, most of the data on the positive effects of LF in pathological conditions are mainly based on studies in animal models. To date, studies in animal models have shown a significant increase in survival in rodents when sepsis developed after injection of
E. coli [
145]. Subsequent work revealed the strong anti-inflammatory effect of LF in models with induced gastritis or enteritis. However, the most promising results come from experiments based on the administration of lactoferrin to subjects with immature digestive systems (possibly due to an underdeveloped intestinal microbiome). Calves and newborn rats were characterized by better absorption of nutrients and a significant increase in intestinal villi length and stimulation of the development of the immune system [
40,
142,
146,
147,
148,
149]. Lactoferrin was reported to protect against oxidative stress-induced mitochondrial dysfunction and DNA damage, thus modulating innate immune responsiveness which can further alter the production of immune regulatory mediators that are important for directing the development of adaptive immune function [
12,
19,
31,
39,
75,
150]. Such LF action was revealed both in cell culture and within an animal model of endotoxemia. In fact, mitochondria from lipopolysaccharide (LPS)-treated animals released significantly higher amounts of H
2O
2 than those isolated from LF-pre-treated plus LPS-challenged animals [
150]. This mechanism is of fundamental protective importance at the beginning of an infection. After the infection phase, lactoferrin shows a strong immunotropic effect: it stimulates the cells of the immune system to mature rapidly and enhance the immune response.
In order to not rely only on animal studies, it is worth recalling clinical trials. Among adults, it was possible to notice an improvement in the condition of people suffering from chronic
H. pylori infection (the most common cause of peptic ulcer disease) in a form resistant to conventional treatment [
151]. The results of studies involving patients suffering from various types of cancer are also promising, although preliminary. The anticancer effects of supplementation with LF in the gastrointestinal tract cancer and protection against colon cancer, stomach cancer, liver cancer and pancreatic cancer may be explained by the antioxidant properties of lactoferrin [
12,
152,
153,
154,
155] (
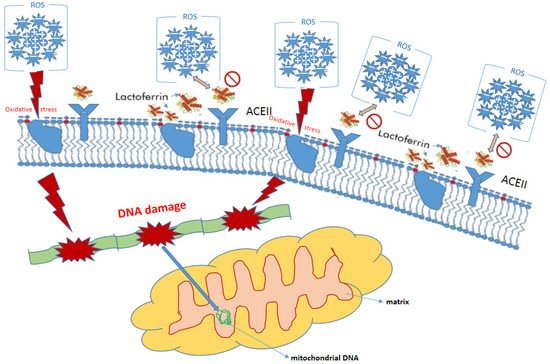
Figure 3).
Figure 3. Protective role of lactoferrin in eukaryotic cell.
Nevertheless, if we consider using LF in therapy, the form of its administration is very important. Lactoferrin is a hydrophilic substance, therefore in the non-liposomal version it has very limited absorption from the stomach [
19]. In free form, it is decomposed there by hydrochloric acid and enzymes (proteases). Therefore, the bioavailability of the free form of lactoferrin may be limited. The use of small liposome vesicles may be beneficial in this case [
156,
157,
158]. Nanoliposomes protect lactoferrin from destruction by digestive juices, allowing the intact protein to pass into the duodenum, from there into the general circulation, ensuring its high bioavailability [
159] and impact on iron ions homeostasis, the skeletal system and, of course, the immune system.
LF administered in a phosphatidylcholine encapsulated form also has the potential to penetrate deep into the mucosa, and due to the small size of the nanoliposome (100 nm) compared to the virus size (150 nm), it is more competitive in reaching receptors on target cells where it settles in front of the virus [
156,
157]. It is important to note that the mucous membranes lining the oral or nasal cavities are very permeable, so this additional protection against viruses based on nanolactoferrin is very relevant.
This entry is adapted from the peer-reviewed paper 10.3390/molecules27092941