1. PMMA
Polymethylmethacrylate (PMMA) bone cement has a long history of use as an antibiotic carrier, with Buchholz and Engelbrecht first publishing on antibiotics mixed with Palacos cement in 1970 [
48]. Structural cement spacers that bear load are an important part of staged revision for PJI. The spacer is inserted following debridement at the first stage of TSE to allow weight-bearing, maintain bony alignment, and aid in mobility and pain management. Local antibiotic elution comes from mixing antibiotics with PMMA before curing, and is a balance between increasing antibiotic concentration and decreasing structural integrity. Antibiotics are available pre-mixed from multiple manufacturers, or may be mixed at the time of surgery. It is effective in reducing infection recurrence, and the use of an antibiotic loaded spacer in TSE is associated with a 7% absolute risk reduction [
49]. Structural spacers have lower antibiotic elution when compared to beads of the same composition [
50]. This is due to beads having greater surface area to volume ratio, with surface elution from PMMA responsible for initial burst levels [
51]. Following this, slower elution is through the network of interconnecting pores and cracks in the material, and passive diffusion through the cement matrix [
52]. Antibiotic beads have been shown to be non-inferior to conventional intravenous antibiotic therapy in osteomyelitis treatment [
53,
54]. In a randomised controlled trial of 28 hip and knee PJI patients, antibiotic beads had a lower infection recurrence rate (15%) compared to conventional parenteral therapy (30%) following prosthesis resection, although this was not statistically significant [
55].
Effective local therapy has questioned the need for prolonged systemic antibiotic treatment in some studies. Stockley et al. provided targeted local antibiotic therapy with PMMA beads at the first stage of 114 TSE for chronic hip PJI [
56]. Patients only received three doses of systemic peri-operative antibiotic prophylaxis, with a success rate of 87.7% at a minimum two-year follow-up. From the same centre, a case series of 53 chronic knee PJIs undergoing TSE or staged arthrodesis reported an infection eradication rate of 89% with use of targeted antibiotics in PMMA beads and spacer [
57]. These patients received a mean of only 4.6 days of intravenous therapy, and only three patients received subsequent oral antibiotics. Hart et al. reported on 48 TSEs for knee PJI using PMMA spacers containing gentamicin and vancomycin, with 88% infection eradication following only two weeks of post-operative intravenous therapy after the first stage [
58].
Variable antibiotic pharmacokinetics have been reported when using PMMA as an antibiotic carrier. Local antibiotic level peaks between day one and three, indicating an initial burst-release followed by a slow decline in concentration; however, the peak levels and length of time above the MIC is variable between studies and difficult to control [
59,
60]. Variability is due to multiple factors, including antibiotic type and concentration, PMMA type, mixing technique, physical wear, use of prefabricated or handmade products, and anatomic location with local fluid turnover [
51,
61]. Nonetheless, peak local antibiotic levels vastly exceed those achievable with systemic therapy [
46]. PMMA antibiotic release is poor, with only 25–50% of contained antibiotic eluted in bead form [
53]. Shaping smaller beads with greater surface area can improve elution, with 3 × 5 mm ‘mini-beads’ releasing 93% of contained antibiotics over 14 days and achieving local concentrations seven times higher than 7 × 7 mm beads [
62].
In PJI, studies that measure antibiotic release from PMMA are predominantly in structural spacers. Some studies demonstrate several months of continuous antibiotic release, while others reveal that local concentrations drop below MIC after seven to fourteen days [
54,
63]. A systematic review of
in-vivo cement spacer antibiotic levels by Anagnostakos and Meyer found no clearly superior cement or antibiotic mix with significant heterogeneity of studies in cement spacer composition and antibiotic level sampling technique [
59]. Invitro studies to define the superior composition of cement in spacers unfortunately have conflicting results. Palacos with gentamicin has shown highest elution in initial studies; however, recent literature has shown other cements to have similar elution kinetics, with variable superiority when changing or adding impregnated antibiotics [
59,
60,
63,
64,
65,
66,
67,
68]. Antibiotic elution may be increased through altering composition by increasing antibiotic concentration or putting other additives in the cement such as glycine, Poly(lactic-co-glycolic) Acid (PLGA), or calcium phosphate [
51,
63,
69]. Stevens et al. demonstrated that spacers with higher antibiotic doses achieved higher burst levels and eluted antibiotics above the MIC for over 80 days [
63].
Mixing technique can alter cement porosity, which subsequently affects antibiotic release. Hand mixing, in comparison to vacuum mixing, can incorporate more air which can increase peak antibiotic concentration up to five-fold in vitro [
65,
70]. Vacuum mixing has a variable effect on antibiotic elution, and in vitro studies confirm improved antimicrobial activity with Cobalt G-HV, Palacos R+G, and Simplex P, while declining performance when using Cemex Genta, Smartset GMV, and Versabond AB [
71]. Using multiple antibiotics can similarly increase porosity and synergistically improve release of both antibiotics [
70]. However, contradicting studies have also found no difference between mixing techniques or with the use of multiple antibiotics [
72,
73].
PMMA may be incompatible with antibiotics due to its effects on the antibiotics themselves, or vice versa. The exothermic polymerisation reaction of PMMA can generate temperatures up to 90 degrees Celsius [
51]. This may degrade contained antibiotics, and heat stability should be considered in antibiotic choice. In vitro, beta-lactams are highly fragile, while gentamicin has only a slight decrease in activity after heat treatment. Other antibiotics including aminoglycosides, glycopeptides, tetracyclines and quinolones are stable over six weeks following initial heat treatment [
74]. Mechanical changes can also occur in PMMA induced by some antibiotics. Antibiotics in liquid form have a greater impact on the structural integrity of cement compared to powdered equivalents, and some antibiotics such as rifampicin cause significant changes in cement consistency during polymerisation resulting in decreased mechanical strength [
51].
Local adverse effects of PMMA appear to be minimal once the cement is cured and the risk of thermal injury has passed. However systemic adverse effects due to supratherapeutic antibiotic levels are being increasingly recognised, especially aminoglycoside nephrotoxicity. Serum levels of gentamicin and vancomycin from spacers are detectable for up to eight weeks [
75], and spacers with high dose vancomycin or aminoglycoside (over 3.6 g antibiotic per 40 g PMMA) have been associated with almost two-fold increase in acute kidney injury (AKI) risk compared to lower dose spacers [
33]. In two systematic reviews, overall rate of AKI following TSE with an antibiotic spacer is 4.18%, compared to 0.55% in primary arthroplasty [
49,
76]. However, this may simply reflect intraoperative hypovolemia and the published studies are clearly susceptible to multiple biases, primarily selection bias. These revision cases are of course far more complicated and prolonged than standard primary arthroplasty, and these results must be interpreted carefully and viewed with caution.
Mechanical studies have demonstrated that PMMA does not appear to lose strength due to elution of antibiotics; however, persistence of the carrier has been associated with the emergence of antibiotic resistance [
77]. Small colony variants emerge following prolonged exposure to subtherapeutic levels of antibiotic, and resistant organisms have been isolated in retrieved cement spacers, although the incidence is unclear [
78]. Persistent cement may also be a focus for clinical infection recurrence [
79]. Removal of PMMA generally requires a second surgery, which carries the risk of local or systemic complications.
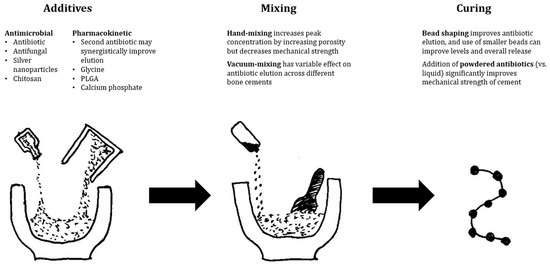
Regardless, PMMA remains an essential antibiotic carrier in the treatment of PJI and is by far the most studied. It is well understood as a biomaterial in orthopaedic surgery due to its long history of use and has effective elution of local antibiotics with proven reduction in infection recurrence in clinical studies. Its ability to bear load is not shared by any other available antibiotic carrier. It is not without issues, with variability in antibiotic levels, incompatibility with several classes of antibiotics, and the requirement for removal following infection clearance. Finally, antibiotic elution from PMMA, despite much lower systemic levels than parenteral therapy, has still been associated with occasional systemic adverse effects. Figure 2 illustrates several evidence-based recommendations from the authors to improve elution characteristics.
Figure 2. Evidence-based recommendations to improve antibiotic elution from PMMA-based antibiotic delivery vehicles.
2. Resorbable Carriers
Resorbable carriers aim to completely degrade to overcome the persistence issues of PMMA and do not require a second surgery for removal. This avoids subtherapeutic levels associated with carrier persistence in PMMA, as all antibiotics are released when the carrier is fully resorbed. In PJI, their resorbable nature makes them more attractive in DAIR or SSE, when there is no planned return to theatre to facilitate removal, as in TSE. Cements or gels may also be mouldable to fit defects, manage surgical dead space and increase antibiotic penetrance.
3. Calcium Sulphate
Calcium sulphate (CS) may be used as an antibiotic carrier, and its effectiveness in chronic osteomyelitis and fracture-related infection has led to further investigation in PJI [
53,
80]. It is promising as a bioabsorbable antibiotic carrier with favourable elution qualities, although it carries a risk of persistent wound leakage, heterotrophic ossification, or life-threatening hypercalcaemia [
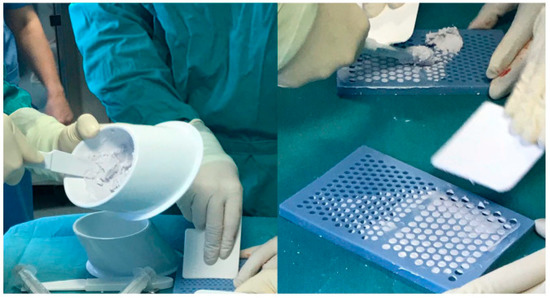
81]. It is available as cement which can be shaped into beads to suit a variety of clinical applications (
Figure 3). It releases 100% of loaded antibiotics over time, and elutes over a period of several weeks [
82]. When combined with targeted antibiotics, it has been reported to successfully inhibit biofilm formation and eradicate established biofilms in vitro in multiple bacterial species [
83]. It has also been shown in vitro to cause minor third-body wear when used in prosthetic joints; however, rates are much lower than with PMMA or ceramic particles [
84]. A systematic review of wound leakage in prosthetic joint surgeries using CS beads revealed a rate of 3.8%, even with deep usage inside the joint, and the included larger studies suggested that higher volumes of CS or medical co-morbidities posed an increased risk of this complication [
81,
85,
86]. A separate systematic review of hypercalcaemia demonstrated a 4.2% overall rate; however, only 0.28% required management, with one case out of 1049 being life-threatening and needing intensive care [
87]. Unfortunately, the comparative studies assessing success rates are limited to case series or case-control studies in DAIR procedures. A matched case-control study of 40 DAIRs for acute hip or knee PJI demonstrated no significant improvement with the use of vancomycin and tobramycin CS beads at 90 days or two years [
88]. This study reported a 45% failure rate, consistent with other smaller published case series of CS DAIRs [
89]. However, a recently published cohort study by Reinisch et al. identified significant improvement in 41 DAIRs for hip PJI, with vancomycin, ceftriaxone, or tobramycin CS beads [
90]. The CS group had a significantly lower revision rate of 15% compared to 64% in the standard group. Studies of CS in SSE or TSE for PJI either do not have re-infection rate as their primary outcome, have no comparator group, or have grouped aseptic and septic revision. Reported re-infection rates range from 0% to 6.7% [
85,
86,
91]. While further higher-quality studies need to be performed, the significant cost and risk of adverse effects of CS carriers should be considered before recommending their routine clinical use in the absence of any demonstrated benefit.
Figure 3. Antibiotic-impregnated Calcium sulphate as cement and shaped into beads (from Ene et al. [
92]).
4. Hydroxyapatite
Calcium hydroxyapatite (CHA) has seen recent renewed interest as an antibiotic carrier, despite first publication of its use over 20 years ago [
92]. A number of calcium apatite compounds are available, including hydroxyapatite and tricalcium phosphate [
92]. These can be prepared as blocks, with an encased antibiotic powder reservoir, or as a cement mixed with antibiotic powder. Isothermic hardening of cements has the advantage of avoiding thermal damage to antibiotics, and its porous nature promotes biological reactivity for bone formation and antibiotic release [
92,
93]. However, the slow and incomplete degradation of these cements causes incomplete and inconsistent antibiotic elution, and promotes bacterial colonisation [
92]. A case series of fourteen knee PJI patients with antibiotic-impregnated CHA pellets (Bone Ceram P, Olympus Terumo Biomaterials Corp, Tokyo, Japan) at the first stage of TSE reported a recurrence rate of 21% at mean follow-up of 5.1 years [
94]. They reported no complications related to use of CHA. However, inconsistent antibiotic elution needs to be addressed before it can be used as a reliable carrier.
Combinations of CHA and CS are more clinically studied in bone and joint infection. Cerament® (BoneSupport AB, Lund, Sweden) is a cement mixture of 40% CHA and 60% CS, and is commercially available with gentamicin or vancomycin (
Figure 4) [
95]. In vitro, the substance has been shown to increase prosthesis pull-out strength and to inhibit biofilm formation with antibiotics [
96,
97]. When used clinically in PJI patients, it can deliver high local antibiotic levels, with drain effluent levels well above MIC at mean 90 h post-op [
98]. However, this study also noted that presence of a post-operative drain significantly reduces available local antibiotic by measuring renal antibiotic clearance. Logoluso et al. found a 95% infection eradication rate with targeted antibiotic-laden Cerament® applied to stemmed components at second-stage of TSE for PJI [
99]. Across two large case series of chronic osteomyelitis, including 71% following infected fracture fixation, McNally et al. reported 94% infection eradication at four to eight years follow-up in higher risk Cierny–Mader type B hosts [
95,
100]. PerOssal® (Osartis GmBH, Dieburg, Germany), a mix of 51.5% nanocrystalline CHA and 48.5% CS, has been used in PJI at first-stage of TSE combined with antibiotics within the intramedullary canal [
101]. The PerOssal group reported a trend towards improved infection recurrence rate (6.67% vs. 16.13%), and significantly lower serum inflammatory marker values from the second to sixth postoperative weeks. There was no difference in complication rate between groups, although they did note delayed wound healing when the substance was used outside of the intramedullary canal. Overall, this combination carrier has significant potential in PJI given its promising results in osteomyelitis; however, similarly to CS, it must be used with caution to avoid local wound healing complications.
5. Bioactive Glass
Bioactive glass (BAG) carries inherent antibacterial activity following implantation and can also act as an antibiotic carrier. It is an attractive option in osteomyelitis and fracture-related infection due to stimulation of bone formation to heal defects, and has been shown to improve local vascularity with proangiogenic properties [
102]. Once implanted, calcium is released into the local environment from the glass, which reacts with interstitial phosphate to form a calcium phosphate layer on the surface of the BAG. Ongoing growth of this layer continues with further dissolution of the glass, which then undergoes crystallisation to hydroxyapatite allowing cellular adhesion [
102,
103,
104]. Sodium and potassium are also released from the substance and exchanged for ionic hydrogen. This increases osmotic pressure and local pH up to 11.65, creating a hostile environment for microbes [
102,
104]. BAG-S53P4 (commercially available as Bonalive®, Turku, Finland) is a silicate glass that provides bactericidal activity against a broad-spectrum of planktonic and biofilm-phase bacteria, including multi-resistant organisms, and also prevents biofilm formation [
105,
106,
107]. In vitro bacterial killing has been shown to be equivalent to antibiotic-loaded PMMA [
108]. Resistance to BAG has not been reported during in vitro studies [
104]. The porous nature of mesoporous and sol-gel glasses allows for release of metal ions and larger molecules, including antibiotics. Metal ions can be incorporated at the time of manufacture and silver, gallium, magnesium, copper, strontium, and zinc have shown positive in vitro results [
103,
104,
109]. Antibiotic compounds have been incorporated into BAG during in vitro studies, with total antibiotic release up to 90%; however, similar to PMMA, heterogeneity exists in both BAG composition and antibiotic used, leading to variability in the level released and the duration [
110,
111,
112,
113,
114]. Newer composite borate glasses exhibit promising elution kinetics compared to silicate glasses and possess tailorable elution qualities based on their composition [
115]. In animal models, antibiotic-loaded borate glass revealed significant improvement in infection clearance and histological tissue quality over glass alone, suggesting that antibiotic therapy provides extra antibacterial activity [
111,
112,
113,
115,
116]. Antibiotic release has been detected in rabbit models out to 21 days for teicoplanin, and fourteen days for vancomycin [
111,
112].
There are no published studies of clinical use of BAG in prosthetic joint infection currently, but non-antibiotic-laden BAG has been used in osteomyelitis treatment following surgical debridement. Lindfors et al. reported an overall cure rate of 89.7% at 31 months in a multinational register of 116 patients, predominantly involving post-traumatic long bone osteomyelitis [
117]. In this study 84.5% of patients received BAG-S53P4 without antibiotics as a single-stage procedure, while 15.5% initially received antibiotic-loaded PMMA beads followed by BAG-S53P4 at the second stage. They also reported significantly more early poor outcomes in the two-stage treatment group. Another prospective study of BAG-S53P4 in 27 cases of long bone osteomyelitis found 88.9% of patients were infection-free at mean 17.8 months follow-up [
106]. A comparative retrospective study of long bone osteomyelitis compared BAG-S53P4 to hydroxyapatite plus calcium sulphate plus antibiotics and tricalcium phosphate plus demineralised bone matrix plus antibiotics [
118]. They found 92.6% of patients who received BAG-S53P4 were infection-free at mean 21.8 months with equivalent infection clearance across the three groups, but significantly lower wound complication rates in the BAG group. Two smaller retrospective case series of eleven and three patients reported infection clearance rates of 91% and 100% after treatment with BAG-S53P4 following debridement [
102,
119]. Importantly, all patients in all studies discussed received systemic antibiotic therapy. Despite these results in the treatment of osteomyelitis, further research is required to understand the utility of bioactive glass in prosthetic joint infection, including its effect on articulated surfaces. Newer composite and borate glasses to deliver antibiotics also have potential to augment the natural antibacterial activity of BAG.
6. Hydrogels
Hydrogels carry significant promise as a fully resorbable carrier that can be tailored to a specific clinical indication. They can be manufactured from multiple biodegradable polymers, with variable antibiotic release kinetics and degradation time of the gel [
120,
121]. Their consistency also makes them versatile for application to implant surfaces or fill dead space, as seen in
Figure 4. They can also carry a wide variety of antimicrobial substances as, in contrast to PMMA, hydrogel can be mixed at room temperature, without thermal damage to the contained antimicrobial. They have been combined in experimental studies or case reports with a variety of antibiotics, antifungals, chitosan, and tyrosol [
122,
123,
124]. The high water content inside the hydrogel matrix is also an appropriate environment for containing and releasing bacteriophages [
125]. Animal models have found that loaded hydrogels are able to achieve the MBEC for multiple common ODRI pathogens [
126]. The forefront of hydrogel technology has produced layered microparticles within a hydrogel to enable multiphase release, in a rabbit model releasing vancomycin and ceftazidime at stable therapeutic level out to 56 days, while simultaneously administering lidocaine for pain relief to 14 days [
127].
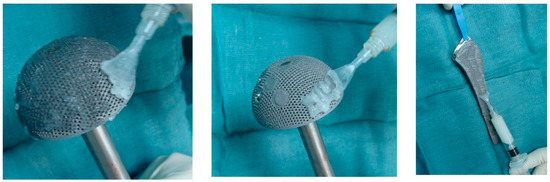
Clinical studies are limited to the use of Defensive Antibacterial Coating (DAC®, Novagenit Srl, Mezzo Lombardo, Italy), which is the most studied and is already commercially available (
Figure 4). DAC® is recommended as a prophylactic implant coating, as it changes the implant surface from hydrophobic to hydrophilic, to impair bacterial adhesion, but is completely resorbed in 72 h [
128]. It can also carry antibiotics to improve its capacity for infection prevention or for PJI treatment. The largest clinical study in PJI treatment is a 1:1 case-control study of cementless TSE by Zagra of 54 patients. At 2.7 years mean follow-up, they reported four recurrences in the control group (14.8%), compared to none in the group which received DAC with targeted antibiotics at the second stage [
129]. Another matched case-control study of 44 patients found equivalence in infection recurrence rate between TSE compared to SSE with DAC and antibiotics, with a significant reduction in hospital stay and antibiotic duration in the DAC group [
130]. However, importantly, all the SSE patients in this study met their criteria for SSE, for which other authors have found equivalent results between SSE and TSE (no large soft tissue defect, previously identified and antibiotic sensitive pathogen) [
131]. In the largest study of DAC use with antibiotics, an RCT of 380 primary and revision arthroplasty patients, the treatment group was not found to have any complications attributable to the hydrogel, with equivalent wound healing, lab biomarkers, and cementless implant osseointegration [
132]. This is consistent with the other smaller clinical and safety studies that have not found any significant complications associated with DAC use. These promising early clinical results for DAC suggest that a dedicated hydrogel for PJI could safely deliver therapeutic levels over a prolonged period.
7. Nanocarriers
Nanocarriers are complex molecular moieties that respond to internal or external stimuli for activation. Self-targeting carriers can respond to local changes in infected tissue, such as pH, hypoxia, macrophage presence, increased reactive oxygen species, local ligands, bacterial enzymes, or increased local temperature [
133]. Nanocarriers can also respond to external stimuli such as photothermal radiation or ultrasound waves [
134,
135]. These carriers, once activated, change their structure from a mobile hydrophilic compound, becoming hydrophobic and releasing their antimicrobial agent. This can be an antibiotic, antifungal, heavy metal nanoparticle, or other bacterio-toxic substance. They can also be designed to release their antimicrobial substance with enhanced pharmacokinetics compared to current options [
133]. Self-propelled ‘Nanorobots’ can be guided using photothermal stimulation to release their antimicrobial substances into a target area [
136].
Figure 4. Defensive Antibacterial Coating (DAC) applied to acetabular and femoral components (from Romano et al. [
132]).
This entry is adapted from the peer-reviewed paper 10.3390/antibiotics12040752