1. Current Difficulties and Challenges
The available evidence still suggests that NAFLD-related cirrhosis is a risk factor for HCC. Though this finding is suggested to be lower than that of HCV-related cirrhosis, the annual incidence of NASH cirrhosis is still higher than 1%. Nevertheless, HCC was also found in NAFLD patients without evidence of cirrhosis, with incidence rates lower than 1% per year. So, more high-quality prospective studies are needed to confirm these findings. This complex and incompletely understood pathogenesis represents significant differences in NAFLD-related HCC and impacts clinical practice. Guidelines recommend HCC surveillance in patients with advanced fibrosis/cirrhosis using abdominal ultrasound every six months [
38]. Although this approach is widely accepted and simple, it needs more cost-effective evidence.
Recent data showed that the “one size fits all” approach is inappropriate, and tailored, individualized risk-based approaches should be implemented [
39]. This should be highlighted more in patients with NAFLD due to its unique characteristics.
Twenty to fifty percent of HCC cases occur in non-cirrhotic patients with underlying NAFLD [
4].
2. Current Techniques
The above difficulties emphasize the importance of using a risk-stratified approach in HCC surveillance, especially in patients with NAFLD. Molecular biomarkers for predicting progressive fibrosis and HCC development in patients with NAFLD are a hot topic with ongoing research. In the era of precision medicine, patients adopt personalized approaches based on their risks and characteristics. In NAFLD-derived HCC, this approach appears interesting, promising, and cost-effective [
43]. In this study, it appeared that using abbreviated MRI in patients with a high risk of HCC development is more cost-effective than the universal approach using ultrasound. The advantages of MRI include its cost-effectiveness if compared to ultrasound regarding targeted diagnosis in high- and intermediate-risk patients due to marked recent reductions in its costs and also, compared to ultrasound, which is usually influenced by the operator’s skills and experience as well as the difficulties that can obstacle the diagnosis, especially in morbid obesity [
8]. In morbid obesity, MRI yields more definite data that will be very important due to the increased and still increasing prevalence of obese patients with nonalcoholic fatty liver diseases. The advances of newly emerged simplified MRI protocols, such as AMRI, are expected to lower the bar for using MRI-based screening to save both money and time [
44].
Moreover, MRI can measure liver fat percentage with the MRI proton density fat fraction (MRI-PDFF). Specifically, chemical shift-encoded (CSE) MRI is one of the best imaging indicators to detect early liver fat deposition [
45]. Non-enhanced MRI is also a good option for HCC surveillance in high-risk patients. Another option is using a new diagnostic algorithm performed by the Japan Society of Hepatology for HCC in patients under surveillance for chronic liver disease, which contemplates the use of gadolinium-ethoxybenzyl-diethylenetriamine pentaacetic acid (EOB-MRI) [
46,
47]. Although expensive and time-consuming, MRI can measure liver fat percentage with magnetic resonance imaging proton density fat fraction (MRI-PDFF). Specifically, chemical shift-encoded (CSE) MRI is the best imaging indicator for early liver fat detection [
48].
Nevertheless, patients who present with compensated advanced liver disease of NASH etiology, particularly obese patients with NASH, have a lower prevalence of portal hypertension compared with other etiologies [
49].
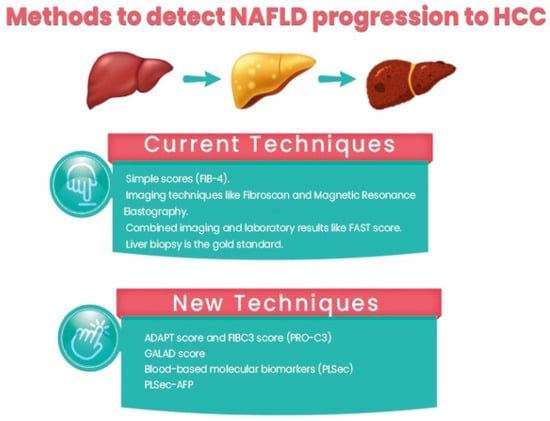
Identifying high-risk patients among those with NAFLD includes identifying patients with a risk of NAFLD progression and advanced fibrosis/cirrhosis and identifying patients with NAFLD cirrhosis with a higher risk of HCC development. Currently, strategies for the identification of patients with a risk of NAFLD progression include:
-
Using simple scores such as the Fibrosis-4 (Fib-4) score, the NAFLD fibrosis score (NFS), the Hepamet fibrosis score (HFS), and the enhanced liver fibrosis (ELF) panel. Among them, Fib-4 is easy and widely used. In a recent study comparing different non-invasive scores, HFS was the best performer for the identification of significant (F0–1 vs. F2–4, AUC = 0.758) and advanced (F0–2 vs. F3–4, AUC = 0.805) fibrosis, while NFS and FIB-4 showed the best performance for detecting histological cirrhosis (range AUCs 0.85–0.88) [
50].
-
Imaging techniques such as Fibroscan and magnetic resonance elastography.
-
Combined imaging and laboratory results such as the FAST score [
51].
-
Liver biopsy is the gold standard for assessment, but due to its invasiveness, sampling errors, and inconvenience in follow-up, its role in clinical practice is limited.
3. What Is New?
N-terminal type III collagen propeptide (PRO-C3) is a marker for collagen formation associated with liver fibrogenesis. So, plasma PRO-C3 levels can correlate with the severity of steatohepatitis and the fibrosis stage. Moreover, the FIBC3 panel is an accurate method with a single threshold value for identifying F ≥ 3 fibrosis in NAFLD with maintained sensitivity and specificity and is superior to other commonly used non-invasive methods. ABC3D is a simplified version that is readily available for use in routine clinical practice. It has also been validated and shown similar accuracy. It can be assessed in serum using ELISA and has recently been used in two promising scoring systems: The ADAPT score [
52] and FIBC3 [
53]. Both showed promising results, better than Fib-4.
The GALAD score is a marker for HCC detection; however, recent data showed that the GALAD score was significantly higher 1.5 years earlier than HCC detection among patients with NAFLD who developed HCC than those who did not, highlighting its potential role as an HCC predictor. Moreover, the GALAD score is superior to individual serum markers for detecting HCC in NASH, regardless of the tumor stage or liver cirrhosis. This can suggest that GALAD should be investigated as a method for screening for NASH [
54]. This approach was mainly based on identifying patients with advanced fibrosis and cirrhosis; however, the risk is not homogenous even in those patients. Non-invasive markers for fibrosis assessment in patients with NAFLD help discriminate between patients with advanced fibrosis and those without fibrosis, but their performance in predicting long-term HCC among patients with advanced fibrosis-cirrhosis is suboptimal [
54]. Blood-based molecular biomarkers (PLSec) were recently identified for HCC prediction in patients with advanced fibrosis-cirrhosis [
55]. PLSec showed good predictive ability for HCC in patients with advanced fibrosis and cirrhosis with different etiologies (adjusted hazard ratio [aHR], 2.35; 95% confidence interval [CI], 1.30–4.23). When combined with AFP, it showed higher predictive ability in two validation cohorts of patients with HCV cure after DAA (HR 3.8) and patients with HCC complete response and HCV cure (HR 3.08). Interestingly, PLSec-AFP performed significantly better in the subgroup of patients with NAFLD/cryptogenic cirrhosis (with an aHR of 11.9). PLSec-AFP is changeable with changes in risk factors such as HCV cure with DAA; thus, it has a potential role as a measurable method for estimating risk change with interventions for HCC prevention [
55]. In this regard, validation of PLSec in NAFLD patients by a large-scale study will soon be a cornerstone of the change in practice (
Figure 1).
Figure 1. Management of NAFLD-related HCC.
4. Selection of Treatment Strategy
To date, different international guidelines do not consider HCC etiology in allocating patients to different therapeutic options, and BCLC is the most widely accepted staging system for HCC. According to the available evidence, NAFLD-related HCC might have a different treatment response than other etiologies. NAFLD-related HCC shows a similar response rate to liver transplantation, trans-arterial chemoembolization, ablation, selective internal radiation therapy, and tyrosine kinase inhibitors [
56,
57], but a better response to resection [
58]. Regarding the surgery for resection, obesity should not be considered a risk factor. It was not associated with any increase in the operative time or increased bleeding. Moreover, laparoscopic liver surgery was feasible in obese patients, similar to lean patients. Increased postoperative morbidity rates were higher in obese patients, but they were mainly related to minor complications, such as abdominal wall infections; liver-specific complications and severe complications were not related to BMI. Good patient selection plays a key role in these circumstances, as in terms of liver function, liver volume, and associated co-morbidities, it is crucial to have good outcomes after liver surgery, independent of BMI [
59,
60].
Immune checkpoint inhibitors are approved as first-line therapy for advanced HCC (BCLC C). Surprisingly, patients with NAFLD-related HCC may have a limited response to immune checkpoint inhibitors compared to HCC due to other etiologies. Several agents of ICI have been approved as first- or second-line treatments for HCC. Analyses of survival outcomes in subgroups evaluating the efficacy of ICIs as first-line treatment revealed a discrepancy between viral HCC and non-viral HCC, including NASH-related HCC. Tremelimumab plus Durvalumab was the only combination of two ICIs tested against sorafenib in the first-line setting, and the only one that showed a relatively higher OS in the non-viral and viral HCC subgroups [
61,
62].
CD8 cells play a critical role in HCC development as they induce NASH-HCC rather than performing immune surveillance. Moreover, it was found that The HCC triggered by anti-PD1 (programmed death-ligand 1) was prevented by depletion of CD8 + T cells or TNF neutralization. Moreover, similar phenotypic and functional profiles in hepatic CD8 + PD1 + T cells were found in patients with NAFLD or NASH. A meta-analysis of three randomized clinical trials examined the inhibitors of PDL1 or PD1 in 1600 patients with advanced HCC, and their results showed that the immune therapy did not improve survival in those patients with non-viral HCC. Other studies showed that patients with NASH-driven HCC treated with anti-PD1 or anti-PDL1 showed poorer survival than patients with HCC due to other etiologies. Overall, these data show that NASH-HCC is less responsive to immunotherapy due to NASH-related abnormal T cell activation that induces tissue damage and leads to impaired immune surveillance [
62].
Although these findings will require validation from larger-scale studies, they may highlight the importance of the etiology as a factor impacting the prognosis of HCC, and future selection of etiology-based therapeutic options may be anticipated.
5. Non-Pharmacological Prevention
HCC prevention mainly depends on managing modifiable risk factors such as viral hepatitis eradication and alcohol cessation. In NAFLD-derived HCC, modifiable risk factors such as DM, obesity, dysbiosis, disease activity, and fibrosis have been established [
61]. The prevention strategy starts by adopting a change in multiple lifestyle factors, which can lead to a significant reduction in HCC incidence in general populations. This highlights the importance of promoting healthy living for the primary prevention of HCC [
62]. Lifestyle interventions and chemoprevention are the main possible methods of HCC prevention in patients with NAFLD [
63]. Weight loss (7% at least) benefits patients with NASH by improving inflammation, ballooning, steatosis, and activity scores [
63]. Despite conflicting evidence regarding fibrosis regression, recent data suggested that 45% of studied NASH patients had fibrosis regression (in paired biopsies with 52-week intervals) after losing 10% of their body weight [
64]. On the other hand, data directly linking weight loss and HCC prevention in patients with NAFLD is lacking. Physical exercise is associated with lower HCC risk independent of weight loss. This was proven by a recent multinational cohort study (HR 0.50; 0.33–0.76) in subjects performing vigorous physical exercise for 2 h twice per week [
65]. The Mediterranean diet is widely recommended in patients with NAFLD due to its beneficial effect on lipid profile, glycemic control, cardiovascular risks, and liver fat contents [
66]. Recently, a systematic review focusing on studies that link the Mediterranean diet and HCC risk showed that adherence to the Mediterranean diet is associated with a reduced risk of primary liver cancer [
66]. The Mediterranean diet has been proven to benefit health, increase longevity, and decrease mortality. This can be performed by exerting anti-inflammatory effects, as it is deficient in saturated fat, refined sugar, and dairy and has a very high content of unsaturated fatty acids, fruits and vegetables, whole grains, and fish. This is similar to the foods consumed in Chinese culture, which are also associated with a lower HCC risk and may be due to the higher consumption of soy products, seafood, traditional soups, and herbal teas [
67].
Multiple studies demonstrated a beneficial effect of regular coffee consumption in patients with chronic liver disease and NASH/NAFLD, decreasing HCC risk [
68,
69]. Although the exact amount is not well defined, a large meta-analysis showed a 35% reduction in the relative risk of HCC development in those who consume two cups of coffee daily [
69]. However, the same meta-analysis failed to demonstrate a significant relative risk reduction in the case of decaffeinated coffee consumption [
70].
6. Chemoprevention
Several drugs were associated with a reduced risk of HCC development in patients with NASH/NAFLD. Growing evidence supports the chemopreventive roles of aspirin, metformin, and statins. Regular administration of 650 mg of aspirin per week was beneficial in reducing 50% of HCC risk in a pooled analysis of two American cohort studies [
71]. Long-term aspirin use was associated with a significant reduction in HCC risk, which is evident after five years of use and is also dose dependent. Those effects were not noted with non-aspirin NSAIDs [
71]. This finding was confirmed in a Swedish study demonstrating a lower HCC risk (HR 0.69; 95% CI 0.62–0.76) in patients who used low-dose aspirin (less than 160 mg daily) for five years without a significant increase in GI bleeding risk [
72]. Metformin is well known to have a chemopreventive role against the development of HCC in patients with DM, and this effect was confirmed in a meta-analysis and extensive population cohort studies. Metformin use in diabetic patients was also shown to lower HCC incidence by 41% to 78% compared to non-metformin users [
73]. Many studies observed the protective role of statins in lowering HCC risk, which was confirmed by a meta-analysis showing a 46% decrease in HCC risk among statin users. It was found that lipophilic statins, such as lovastatin or simvastatin, are more beneficial than hydrophilic statins, such as pravastatin, for HCC. Moreover, it has shown better outcomes in the Western population than in the Asian population. Genetic variations in the structure or polymorphism between Asian and Western people may affect statins’ pharmacokinetics and pharmacodynamic properties [
74]. Another meta-analysis confirmed this beneficial effect for lipophilic statins more than hydrophilic statins (51% versus 27%) [
75].
The recent advances in molecular biomarkers as measurable predictors of HCC risk, such as PLSec AFP, may provide a method to guide future research by using them as an endpoint for preventive interventions. The blood-based PLSec-AFP sharply stratifies patients with advanced liver fibrosis who are at risk for long-term HCC and, therefore, gives a guide to risk-based, tailored HCC screening. This approach will identify higher-risk patients who may benefit more from interventional preventive measures. Moreover, this approach may offer a re-evaluation of individual risk after preventive interventions and the need to switch from one to another [
55].
Radioembolization (RE) has been proven from retrospective studies to be effective for the treatment of locally advanced HCC with fewer side effects in comparison to systemic therapy. RE can be a safe option in the treatment strategy, especially in the case of potential contraindications for standard loco-regional and systemic treatments [
56].
This entry is adapted from the peer-reviewed paper 10.3390/diagnostics13162631