Hydrogels can be considered as mimics of the extracellular matrix (ECM). Through integrins, the cytoskeleton is connected to the ECM, and cytoskeleton tension depends on ECM stiffness. A number of age-related diseases depend on cellular processes related to cytoskeleton function. Some examples of cancer initiation and progression and heart disease in relation to ECM stiffness have been analyzed. The incorporation of rigid particles into the ECM can increase ECM stiffness and promote the formation of internal residual stresses. Water migration, changes in water binding energy to biomactomolecules, and changes in the state of water from tightly bound water to free and loosely bound water lead to changes in the stiffness of the ECM.
1. Introduction
Hydrogels can be used as artificial extracellular matrices in tissue engineering in the development of scaffolds for biomimetic three-dimensional cell culture.
The design of hydrogels with tunable physiochemical and biological properties and their potential applications in regenerative medicine were discussed by researchers from Harvard University and Massachusetts Institute of Technology in 2014 [
3]. Hydrogels resemble the native extracellular matrix, and their use as “three-dimensional (3D) matrices for tissue engineering, drug-delivery vehicles, composite biomaterials, and as injectable fillers in minimally invasive surgeries” was characterized as promising for future applications.
2. Advantages and Risks of Nanocomposite Hydrogels
2.1. Cardiac Tissue Engineering
The application of hydrogels in the repair and regeneration of damaged hearts was discussed [
6,
7,
8].
Researchers at the University of Colorado Boulder discussed the three-dimensional encapsulation of adult mouse cardiomyocytes in hydrogels with tunable stiffness [
6]. The morphology and the function of the cardiomyocytes depended on the mechanical properties of their microenvironment. The authors used photo-clickable thiol-ene poly (ethylene glycol) (PEG) hydrogels for the three-dimensional cell culture of adult mouse cardiomyocytes. The stiffness of the PEG hydrogels could be tuned to mimic the microenvironments of cells, and cell function was dependent on the PEG hydrogel stiffness.
Injectable conductive carbon and metal-based nanocomposite hydrogels for cardiac tissue engineering were reviewed in [
8]. It would be beneficial in the near future to study the role and impact of nanoparticle rigidity quantitatively and in more detail, especially in the context of mechanical moduli improvement and in cardiac tissue engineering.
2.2. Multi-Layer Polyelectrolyte Complex Hydrogels and Bioprinting
Multi-layered hydrogels prepared using layer-by-layer self-assembly can be used for biomedical applications [
17].
Layer-by-layer-assembled drug delivery systems can be developed and applied for cancer treatment and in cardiovascular surgery for the surface functionalization of various implants. LbL assembly of soft polymers on nanoparticles can decrease the risks of adverse effects of rigid particles on biological processes caused by high stiffness. The advantage of LbL assembly technology is that it allows controlling the thickness and surface charge of oppositely charged polyelectrolytes near the rigid surface by tuning the assembling conditions. Electrostatic force, hydrogen bonding, charge transfer interaction, and covalent bonding play a role in LbL assembly. Various synthetic, such as doxorubicin or paclitaxel, and natural, such as curcumin, drugs for cancer treatment and their combinations can be loaded into different layers of polyelectrolyte multilayers.
Polyelectrolyte multilayers with tunable hydration and stiffness also have potential to be used in drug-eluting stents as tailored blood-contacting coatings and in novel atraumatic surgical instruments. The prevention of in-stent restenosis and thrombosis also has potential to be achieved by using polyelectrolyte multilayers.
2.3. Residual Internal Stresses
The residual tensile and compressive stresses arise near filler particles in polymer composites [
20]. Due to mechanotransduction, such mechanical stresses arise similarly in the ECM and can be transferred into cytoskeleton tension with the activation of the NF-κB signaling pathway and changing gene expression. The presence of soft components at small concentrations in the rigid matrix results in the development of compressive stress due to differences in the thermal expansion coefficients and leads to maximum dependence of the elasticity modulus on the filler concentration. The temperature changes result in changes of the residual stresses values and reinforcement values. The migration of low-molecular-weight molecules with higher thermal expansion coefficients to interfaces can increase ECM stiffness due to the development of compressive residual stresses.
The stresses and properties of a composite gel consisting of a poly(vinyl alcohol (PVA) matrix filled with poly(acrylic acid) (PAA) microgel particles were reported [
21]. The swelling of the PAA particles was limited by the tensile stress developing in the PVA matrix. The maximum tensile stress was found to increase with the stiffness of the PVA matrix and to decrease with increasing crosslink density of the PAA.
Cells respond to mechanical microenvironment cues via Rho signaling [
22]. Rho GTPase is closely related to tumorigenesis, invasion, and metastasis. The Rho subfamily is involved in the formation of tensile fibers and focal adhesion complexes (FACs). The Rho signaling pathway plays an important role in the development of cancer, and it is also expected to be possible treat cancer by developing inhibitors of the Rho signaling pathway [
23].
2.4. Viscoelastic Hydrogels
A group of authors from Stanford University, Harvard University, The University of Queensland, and the University of Pennsylvania discussed the effect of ECM viscoelasticity in hydrogels on cellular behavior [
24].
Viscoelastic hydrogels with tunable stress relaxation were developed, and the stress relaxation was shown to regulate cell differentiation, spreading, and proliferation [
25]. The faster relaxation in alginate-PEG hydrogels was associated with increased spreading and proliferation of fibroblasts and enhanced osteogenic differentiation of mesenchymal stem cells (MSCs). It can be considered that stress relaxation is related to residual stresses [
20].
It has been reported that stress relaxation of semi-flexible chain segments in hydrogels made from the polyelectrolyte complexation of sodium hyaluronate (HA) and chitosan
increases with increasing temperature and salt concentration [
26].
2.5. Hydrogel Nanocomposites
It has been reported in an article published by researchers from the University of Cambridge that hydrogels resemble the ECM and can support cell proliferation when they are used as tissue engineering scaffolds and that nanofibers improve both the mechanical properties and biofunctionality of hydrogels [
28].
However, it still must be taken into consideration that nanofibers localized in the ECM can increase viscosity and stiffness due to the presence of rigid surfaces at interfaces of the heterogeneous matrix and initiate harmful processes in cells related to the initiation of severe age-related diseases through mechanotransduction. Therefore, the functionality improvement must be confirmed in additional studies.
The interaction between the ECM and nanoparticles was discussed [
30]. It was reported that the risk of toxic effects of nanoparticles increases with decreasing particle size. Nanoparticle-dependent signaling can alter the ratio between ECM-degrading enzymes called matrix metalloproteinases (MMPs) and tissue inhibitors of matrix proteases (TIMPs), resulting in the facilitation of inflammatory processes and ECM pathological changes.
The extracellular matrix stiffness regulates the induction of malignant phenotypes in the mammary epithelium. Researchers at Stanford University confirmed that cells sense the stiffness of their surrounding ECM through Rho-mediated contraction of the actin-myosin cytoskeleton [
31].
2.6. Effect of Water
The dehydration, glycation, crosslinking, and redistribution of bound water molecules and entropy-driven release of tightly bound water molecules are the processes leading to ECM stiffening [
27,
34,
35,
36,
37,
38].
Enhanced mobility of intracellular water for cancer cells vs. non-malignant cells investigated using quasi-elastic neutron scattering (QENS) was reported [
39]. The binding energy of water molecules to biomacromolecules decreases in cancer cells, and it is possible to suggest that a decrease in binding energy results in the transformation of a healthy cell into a cancer cell. The role of water binding energy in the initiation of various diseases was reviewed in [
35,
36,
37].
Water response to the anticancer drug cisplatin was investigated using neutron scattering spectroscopy performed at the ISIS Pulsed Neutron and Muon Source of the Rutherford Appleton Laboratory in a low-energy OSIRIS high-flux indirect-geometry time-of-flight spectrometer, and intracellular water mobility reduction was observed [
40].
It has been concluded that normal-to-malignant transformation is a poorly understood process associated with and dependent on the dynamical behavior of water, so two different water populations were considered: “one displaying bulk-like dynamics (extracellular and intracellular/cytoplasmic) and another with constrained flexibility (extracellular/interstitial and intracellular/hydration layers)” [
41].
2.7. ECM Stiffness and Cancer
2.7.1. Cancer Models
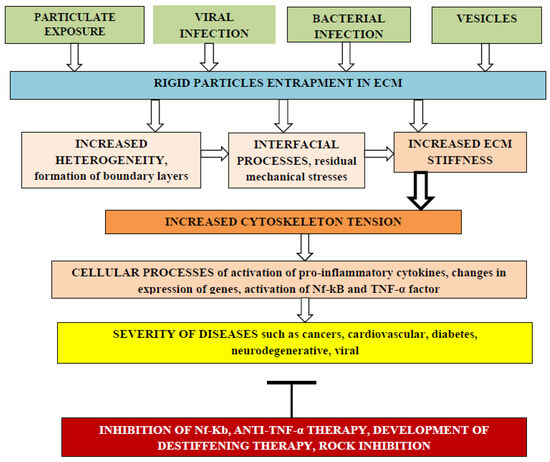
Cellular biochemical processes depend on ECM mechanical stiffness. Entrapment of rigid particles can enhance ECM stiffness due to interfacial changes near rigid surfaces and cause various diseases including initiation and progress of different cancers, as shown in Figure 1.
Figure 1. The effect of rigid particle entrapment by the ECM on the risk of adverse cellular processes that increase severity of various diseases. Different kinds of rigid particles such as nanoengineered particles, particles from air pollution, viruses, bacteria, and extracellular vesicles can be entrapped by the ECM, changing matrix heterogeneity due to the formation of interfacial regions with different elasticity moduli and thermal expansion coefficients. Internal residual tensile and compressive mechanical stresses can arise in the ECM, leading to enhanced stiffness that can be transmitted into cells through integrins, increasing cytoskeleton tension with activation of pro-inflammatory cytokines, changes in expression of genes, activation of the Nf-kB pathway, and expression of TNF-α and IL-6 pro-inflammatory factors. Such cellular processes increase the severity of various diseases and stimulate the development of adverse process inhibition methods.
Cancer development and progression can be associated with remodeling of the ECM. The layer-by-layer coating terminated by hyaluronic acid on calcium carbonate nanoparticles can be applied for targeting breast cancer cells. Hyaluronic acid (HA) is known as a ligand of the tumor-associated receptor CD44 [
44]. The coating slows down the release of the encapsulated model drug. The authors concluded that future studies should focus on understanding the role of different HA receptors in nanoparticles’ accumulation in the extracellular matrix.
Cells regulate the structure and performance of the ECM; for example, cells secrete biomolecules that diffuse into the ECM, creating temporally and spatially controlled gradients that play an important role in inflammation and cancer [
45]. HA is a main component of the ECM.
Hydrogels with various stiffness have been designed, and their influence on the morphology of mesenchymal stem cells (MSCs) and their differentiation was studied [
47]. An artificial ECM for tumorigenesis research applications was reviewed in [
48].
It was shown that the time-dependent stiffness of methacrylated hyaluronic acid hydrogels can be dynamically modulated from “normal” (<150 Pascals) to “malignant” (>3000 Pascals) during an investigation of cultured mammary epithelial cells (MECs) in order to mimic and understand breast cancer development [
49].
The inhibition of the Wnt/beta-catenin pathway in colon cancer cells by the most active vitamin D metabolite, 1alpha,25-dihydroxyvitamin D3 (1,25(OH)2D3), was reported. It was suggested that 1,25(OH)2D3 distinctly regulates two genes encoding the extracellular Wnt inhibitors DICKKOPF-1 (DKK-1) and DICKKOPF-4 (DKK-4). 1,25(OH)2D3 increases the expression of DKK-1, which acts as a tumor suppressor in human colon cancer cells and represses DKK-4 transcription, which is up-regulated in colorectal tumors, increasing cell migration and invasion [
53,
54].
2.7.2. Polyphenols Decrease ECM Stiffness
The effect of polyphenols on the microbiome has been reviewed in [
62]. Polyphenols can inhibit NF-κB and TNF-α, which lead to the increase in tissue stiffness and permeability [
63]. At least one aromatic ring with a hydroxyl group attached to it was mentioned as a common feature for polyphenols [
64]. The polymers with such chemical structure can also be recommended for use as matrix plasticizers.
The beneficial health effect of polyphenols can be explained by their potential de-stiffening of the ECM due to the plasticizing effect that has been earlier demonstrated on edible polymer films [
65]. The essential decrease in Young’s modulus was reported when gallic acid, p-hydroxy benzoic acid, and ferulic acid were used as plasticizers, but the lack of any considerable plasticizing effect for flavon was observed.
The antiplasticizing effect of tannic acid incorporated into films from sorghum kafirin was observed with reduced elongation but increased tensile strength and Young’s modulus. The antiplasticizing effect could be due to the increased number of hydroxyl groups tightly binding within the matrix [
66].
Curcumin upregulates Nrf2 nuclear translocation [
67,
68]. The activation of Nrf2 by curcumin can inhibit the activation of tissue stiffness, inflammatory cytokine expression, and the NF-kB pathway [
69,
70].
Dietary polyphenols can decrease arterial stiffness [
62], and it was reported that curcumin downregulates the pro-inflammatory cytokines TNF-α and IL-6 [
71]. Thus, the joint consideration of these two articles allows arriving at the conclusion that ECM de-stiffening downregulates the expression of pro-inflammatory cytokines, and it has recently been reported that matrix stiffness exacerbates the pro-inflammatory responses of vascular smooth muscle cells [
72].
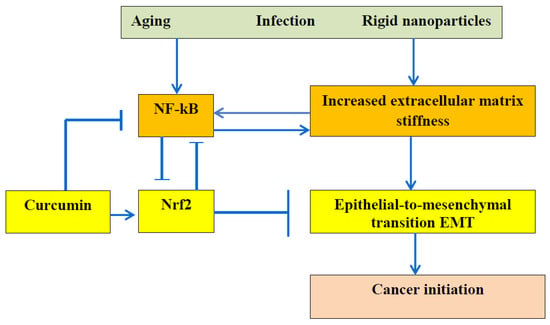
The relationship between the ECM stiffness and EMT is presented in Figure 2.
Figure 2. It is commonly accepted that dysregulation of NF-κB can lead to autoimmune diseases, chronic inflammation, and cancer. This scheme demonstrates the especially important role of ECM stiffness. Advanced age, bacterial and viral infection, and particles from air pollution reinforce the ECM, and increased stiffness activates the NF-kB pathway and causes epithelial-to-mesenchymal transition, leading to cancer initiation. The polyphenol curcumin can inhibit NF-κB due to Nrf2 activation and can retard cancer initiation.
2.7.3. Chitosan and Chitosan Oligosaccharides
The use of injectable in situ chitosan hydrogels in cancer treatment was reviewed in [
78]. It is generally believed that cancers may result from the interaction between healthy cells and carcinogens such as ultraviolet (UV) radiation, chemical carcinogens such as asbestos and tobacco smoke, and biological carcinogens such as infections by viruses and contamination of food by mycotoxins. The authors considered that external agents cause genetic alterations resulting in mutations and thereby perturbing normal cell function. But the effect of the above-mentioned agents on the ECM must also be taken into consideration.
Chitosan-based hydrogels possess the required good biocompatibility, mucosal adhesion, and hemostatic activity with potential for application in tissue engineering and drug delivery [
79]. The fact that extracellular pH values in tumors are lower than in healthy tissues (pH = 7.4) [
80] can be used in drug delivery systems. An injectable chitosan-chondroitin sulfate hydrogel with embedded kartogenin-loaded microspheres as an ultrasound-triggered drug delivery system for cartilage tissue engineering was designed and fabricated.
2.7.4. Anti-TNF-α and Anti-NF-κB Therapies
Anti-TNF-α therapy decreases ECM stiffness and can be used in the treatment of age-related diseases such as rheumatoid arthritis [
38,
63,
82]. The nuclear factor-κB (NF-κB) signaling pathway is involved in inflammation through the regulation of cytokines’, chemokines’, and adhesion molecules’ expression.
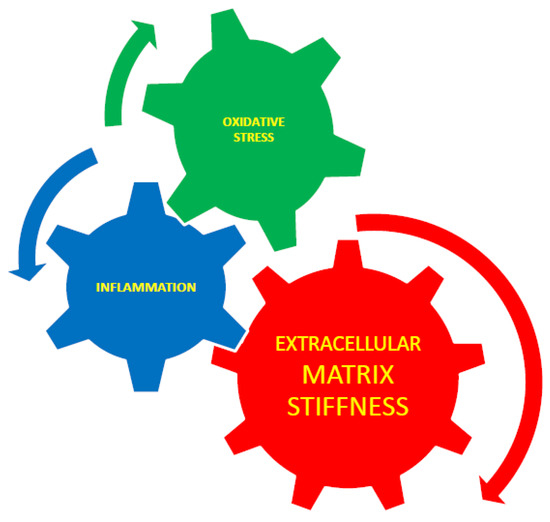
2.8. Equivalence of ECM Stiffness, Oxidative Stress, and Inflammation
The NF-kB pathway is identified as one of the main inflammatory pathways.
It has been reported that oxidative stress initiates arterial stiffness [
86] and that stiffness can initiate oxidative stress [
87]. Therefore, it can be suggested that oxidative stress and tissue stiffening are not two separate processes but two different aspects of a united process measured using different methods. The interaction between extracellular matrix stiffness, inflammation, and oxidative stress is presented schematically in
Figure 3.
Figure 3. The interaction between extracellular matrix stiffness, inflammation, and oxidative stress. Extracellular matrix stiffness plays an essential role in the control of inflammation and cytoskeletal rearrangement, and it is known that inflammation causes stiffness [
63] through TNF-α-dependent vascular inflammation. ECM stiffness is interrelated with oxidative stress. Inflammation and oxidative stress are also interrelated [
38].
Angiotensin-converting enzyme inhibitors (ACEIs) decrease arterial stiffness [
88]. The role of the renin–angiotensin–aldosterone system (RAAS) in the inhibition of arterial stiffness was discussed. The excessive stimulation of angiotensin type 1 (AT1) receptors leads to oxidative stress and vascular inflammation. RAAS inhibition decreases arterial stiffness [
89]. The effects of two RAAS inhibitors (quinapril and aliskiren) and two beta-blockers (atenolol and nebivolol) on arterial stiffness, measured using pulse wave velocity (PWV), have been reported [
90].
This entry is adapted from the peer-reviewed paper 10.3390/gels9090754