Sirtuins belong to the class III histone deacetylases and possess nicotinamide adenine dinucleotide-dependent deacetylase activity. They are involved in the regulation of multiple signaling pathways implicated in cardiovascular diseases. Autophagy is a crucial adaptive cellular response to stress stimuli. Mounting evidence suggests a strong correlation between Sirtuins and autophagy, potentially involving cross-regulation and crosstalk. Sirtuin-mediated autophagy plays a crucial regulatory role in some cardiovascular diseases, including atherosclerosis, ischemia/reperfusion injury, hypertension, heart failure, diabetic cardiomyopathy, and drug-induced myocardial damage.
- Sirtuins
- autophagy
- FOXOs
- AMPK
- mTOR
- cardiovascular diseases
1. Atherosclerosis
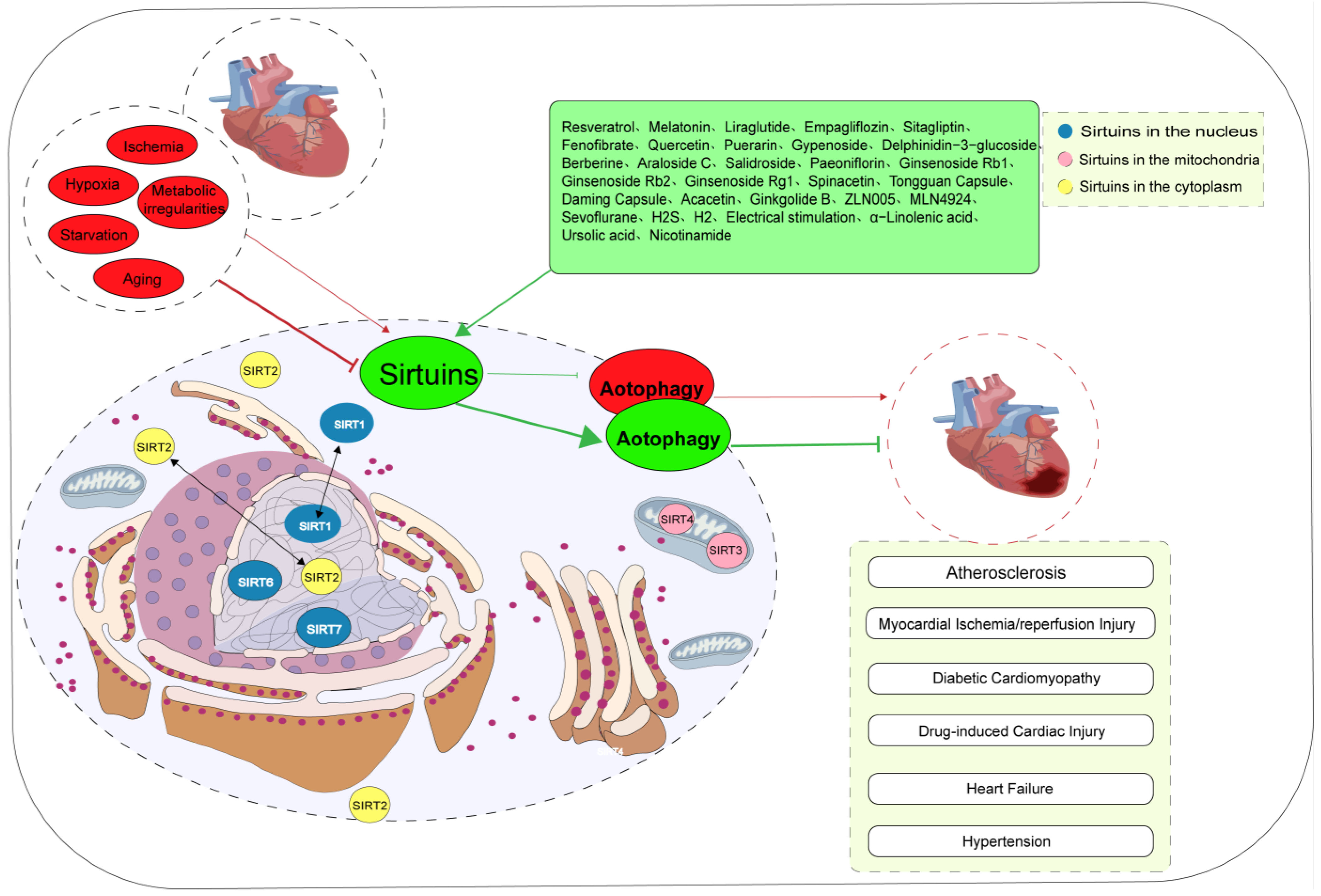
2. Myocardial Ischemia/Reperfusion Injury
3. Diabetic Cardiomyopathy
4. Drug-Induced Cardiac Injury
5. Heart Failure
6. Hypertension
7. Cardiogenesis and Cardiac Maintenance
This entry is adapted from the peer-reviewed paper 10.3390/jcdd10090382
References
- Grootaert, M.O.J.; Bennett, M.R. Sirtuins in atherosclerosis: Guardians of healthspan and therapeutic targets. Nat. Rev. Cardiol. 2022, 19, 668–683.
- Kitada, M.; Ogura, Y.; Koya, D. The protective role of Sirt1 in vascular tissue: Its relationship to vascular aging and atherosclerosis. Aging 2016, 8, 2290–2307.
- Luo, Y.; Lu, S.; Gao, Y.; Yang, K.; Wu, D.; Xu, X.; Sun, G.; Sun, X. Araloside C attenuates atherosclerosis by modulating macrophage polarization via Sirt1-mediated autophagy. Aging 2020, 12, 1704–1724.
- Hassanpour, M.; Rezabakhsh, A.; Pezeshkian, M.; Rahbarghazi, R.; Nouri, M. Distinct role of autophagy on angiogenesis: Highlights on the effect of autophagy in endothelial lineage and progenitor cells. Stem Cell Res. Ther. 2018, 9, 305.
- Wang, C.; Mao, C.; Lou, Y.; Xu, J.; Wang, Q.; Zhang, Z.; Tang, Q.; Zhang, X.; Xu, H.; Feng, Y. Monotropein promotes angiogenesis and inhibits oxidative stress-induced autophagy in endothelial progenitor cells to accelerate wound healing. J. Cell. Mol. Med. 2018, 22, 1583–1600.
- Li, Y.; Cui, W.; Song, B.; Ye, X.; Li, Z.; Lu, C. Autophagy-Sirtuin1(SIRT1) Alleviated the Coronary Atherosclerosis (AS)in Mice through Regulating the Proliferation and Migration of Endothelial Progenitor Cells (EPCs) via wnt/β-catenin/GSK3β Signaling Pathway. J. Nutr. Health Aging 2022, 26, 297–306.
- Wu, Q.; Hu, Y.; Jiang, M.; Wang, F.; Gong, G. Effect of Autophagy Regulated by Sirt1/FoxO1 Pathway on the Release of Factors Promoting Thrombosis from Vascular Endothelial Cells. Int. J. Mol. Sci. 2019, 20, 4132.
- Ma, S.; Chen, J.; Feng, J.; Zhang, R.; Fan, M.; Han, D.; Li, X.; Li, C.; Ren, J.; Wang, Y.; et al. Melatonin Ameliorates the Progression of Atherosclerosis via Mitophagy Activation and NLRP3 Inflammasome Inhibition. Oxid. Med. Cell. Longev. 2018, 2018, 9286458.
- He, J.; Zhang, G.; Pang, Q.; Yu, C.; Xiong, J.; Zhu, J.; Chen, F. SIRT6 reduces macrophage foam cell formation by inducing autophagy and cholesterol efflux under ox-LDL condition. FEBS J. 2017, 284, 1324–1337.
- Wang, T.; Sun, C.; Hu, L.; Gao, E.; Li, C.; Wang, H.; Sun, D. Sirt6 stabilizes atherosclerosis plaques by promoting macrophage autophagy and reducing contact with endothelial cells. Biochem. Cell Biol. 2020, 98, 120–129.
- Zhao, Y.; Jia, X.; Yang, X.; Bai, X.; Lu, Y.; Zhu, L.; Cheng, W.; Shu, M.; Zhu, Y.; Du, X.; et al. Deacetylation of Caveolin-1 by Sirt6 induces autophagy and retards high glucose-stimulated LDL transcytosis and atherosclerosis formation. Metabolism 2022, 131, 155162.
- Zi, Y.; Yi-An, Y.; Bing, J.; Yan, L.; Jing, T.; Chun-Yu, G.; Fan, P.; Hao, L.; Jia-Ni, T.; Han-Jin, H.; et al. Sirt6-induced autophagy restricted TREM-1-mediated pyroptosis in ox-LDL-treated endothelial cells: Relevance to prognostication of patients with acute myocardial infarction. Cell Death Discov. 2019, 5, 88.
- Yuan, P.; Hu, Q.; He, X.; Long, Y.; Song, X.; Wu, F.; He, Y.; Zhou, X. Laminar flow inhibits the Hippo/YAP pathway via autophagy and SIRT1-mediated deacetylation against atherosclerosis. Cell Death Dis. 2020, 11, 141.
- Su, G.; Yang, W.; Wang, S.; Geng, C.; Guan, X. SIRT1-autophagy axis inhibits excess iron-induced ferroptosis of foam cells and subsequently increases IL-1Β and IL-18. Biochem. Biophys. Res. Commun. 2021, 561, 33–39.
- Garlick, P.B.; Davies, M.J.; Hearse, D.J.; Slater, T.F. Direct detection of free radicals in the reperfused rat heart using electron spin resonance spectroscopy. Circ. Res. 1987, 61, 757–760.
- Liu, Y.; Zhang, J.; Zhang, D.; Yu, P.; Zhang, J.; Yu, S. Research Progress on the Role of Pyroptosis in Myocardial Ischemia-Reperfusion Injury. Cells 2022, 11, 3271.
- Koltai, M.; Tosaki, A.; Hosford, D.; Braquet, P. Ginkgolide B protects isolated hearts against arrhythmias induced by ischemia but not reperfusion. Eur. J. Pharmacol. 1989, 164, 293–302.
- Toldo, S.; Mauro, A.G.; Cutter, Z.; Abbate, A. Inflammasome, pyroptosis, and cytokines in myocardial ischemia-reperfusion injury. Am. J. Physiol. Heart Circ. Physiol. 2018, 315, H1553–H1568.
- Sinning, C.; Westermann, D.; Clemmensen, P. Oxidative stress in ischemia and reperfusion: Current concepts, novel ideas and future perspectives. Biomark. Med. 2017, 11, 11031–11040.
- Morales, C.R.; Pedrozo, Z.; Lavandero, S.; Hill, J.A. Oxidative stress and autophagy in cardiovascular homeostasis. Antioxid. Redox Signal. 2014, 20, 507–518.
- Chen-Scarabelli, C.; Agrawal, P.R.; Saravolatz, L.; Abuniat, C.; Scarabelli, G.; Stephanou, A.; Loomba, L.; Narula, J.; Scarabelli, T.M.; Knight, R. The role and modulation of autophagy in experimental models of myocardial ischemia-reperfusion injury. J. Geriatr. Cardiol. 2014, 11, 338–348.
- Lazou, A.; Iliodromitis, E.K.; Cieslak, D.; Voskarides, K.; Mousikos, S.; Bofilis, E.; Kremastinos, D.T. Ischemic but not mechanical preconditioning attenuates ischemia/reperfusion induced myocardial apoptosis in anaesthetized rabbits: The role of Bcl-2 family proteins and ERK1/2. Apoptosis 2006, 11, 2195–2204.
- Yan, H.F.; Tuo, Q.Z.; Yin, Q.Z.; Lei, P. The pathological role of ferroptosis in ischemia/reperfusion-related injury. Zool. Res. 2020, 41, 220–230.
- Li, H.; Zheng, F.; Zhang, Y.; Sun, J.; Gao, F.; Shi, G. Resveratrol, novel application by preconditioning to attenuate myocardial ischemia/reperfusion injury in mice through regulate AMPK pathway and autophagy level. J. Cell. Mol. Med. 2022, 26, 4216–4229.
- Luo, G.; Jian, Z.; Zhu, Y.; Zhu, Y.; Chen, B.; Ma, R.; Tang, F.; Xiao, Y. Sirt1 promotes autophagy and inhibits apoptosis to protect cardiomyocytes from hypoxic stress. Int. J. Mol. Med. 2019, 43, 2033–2043.
- Zhong, Z.; Luo, X.Y.; Xiang, P.; Ji, H.H.; Wu, X.D.; Chong, A.G.; Hu, X.Y.; Cao, X.L. MRTF-A alleviates myocardial ischemia reperfusion injury by inhibiting the inflammatory response and inducing autophagy. Mol. Cell. Biochem. 2023, 478, 343–359.
- Zweier, J.L.; Flaherty, J.T.; Weisfeldt, M.L. Direct measurement of free radical generation following reperfusion of ischemic myocardium. Proc. Natl. Acad. Sci. USA 1987, 84, 1404–1407.
- Blasig, I.E.; Ebert, B.; Hennig, C.; Pali, T.; Tosaki, A. Inverse relationship between ESR spin trapping of oxyradicals and degree of functional recovery during myocardial reperfusion in isolated working rat heart. Cardiovasc. Res. 1990, 24, 263–270.
- Zheng, Y.; Shi, B.; Ma, M.; Wu, X.; Lin, X. The novel relationship between Sirt3 and autophagy in myocardial ischemia-reperfusion. J. Cell. Physiol. 2019, 234, 5488–5495.
- Wu, D.; Ji, H.; Du, W.; Ren, L.; Qian, G. Mitophagy alleviates ischemia/reperfusion-induced microvascular damage through improving mitochondrial quality control. Bioengineered 2022, 13, 3596–3607.
- Ma, L.L.; Kong, F.J.; Dong, Z.; Xin, K.Y.; Wang, X.X.; Sun, A.J.; Zou, Y.Z.; Ge, J.B. Hypertrophic Preconditioning Attenuates Myocardial Ischaemia-Reperfusion Injury by Modulating SIRT3-SOD2-mROS-Dependent Autophagy. Cell Prolif. 2021, 54, e13051.
- Gao, R.; Lv, C.; Qu, Y.; Yang, H.; Hao, C.; Sun, X.; Hu, X.; Yang, Y.; Tang, Y. Remote Ischemic Conditioning Mediates Cardio-protection After Myocardial Ischemia/Reperfusion Injury by Reducing 4-HNE Levels and Regulating Autophagy via the ALDH2/SIRT3/HIF1α Signaling Pathway. J. Cardiovasc. Transl. Res. 2023, 1–14.
- Li, J.; Lu, K.; Zhang, X.; Wang, T.; Li, Q.; Yu, X.; Han, W.; Sun, L. SIRT3-mediated mitochondrial autophagy in refeeding syndrome-related myocardial injury in sepsis rats. Ann. Transl. Med. 2022, 10, 211.
- Araki, S.; Izumiya, Y.; Rokutanda, T.; Ianni, A.; Hanatani, S.; Kimura, Y.; Onoue, Y.; Senokuchi, T.; Yoshizawa, T.; Yasuda, O.; et al. Sirt7 Contributes to Myocardial Tissue Repair by Maintaining Transforming Growth Factor-β Signaling Pathway. Circulation 2015, 132, 1081–1093.
- Wang, X.; Xu, Y.; Li, L.; Lu, W. Thrombin Aggravates Hypoxia/Reoxygenation Injury of Cardiomyocytes by Activating an Autophagy Pathway-Mediated by SIRT1. Med. Sci. Monit. 2021, 27, e928480.
- Jia, G.; Hill, M.A.; Sowers, J.R. Diabetic Cardiomyopathy: An Update of Mechanisms Contributing to This Clinical Entity. Circ. Res. 2018, 122, 624–638.
- Dewanjee, S.; Vallamkondu, J.; Kalra, R.S.; John, A.; Reddy, P.H.; Kandimalla, R. Autophagy in the diabetic heart: A potential pharmacotherapeutic target in diabetic cardiomyopathy. Ageing Res. Rev. 2021, 68, 101338.
- Kanamori, H.; Takemura, G.; Goto, K.; Tsujimoto, A.; Mikami, A.; Ogino, A.; Watanabe, T.; Morishita, K.; Okada, H.; Kawasaki, M.; et al. Autophagic adaptations in diabetic cardiomyopathy differ between type 1 and type 2 diabetes. Autophagy 2015, 11, 1146–1160.
- Qiu, Z.; Ming, H.; Zhang, Y.; Yu, Y.; Lei, S.; Xia, Z.Y. The Protective Role of Bmal1-Regulated Autophagy Mediated by HDAC3/SIRT1 Pathway in Myocardial Ischemia/Reperfusion Injury of Diabetic Rats. Cardiovasc. Drugs Ther. 2022, 36, 229–243.
- Yu, W.; Gao, B.; Li, N.; Wang, J.; Qiu, C.; Zhang, G.; Liu, M.; Zhang, R.; Li, C.; Ji, G.; et al. Sirt3 deficiency exacerbates diabetic cardiac dysfunction: Role of Foxo3A-Parkin-mediated mitophagy. Biochim. Biophys. Acta. Mol. Basis Dis. 2017, 1863, 1973–1983.
- Guo, Z.; Tuo, H.; Tang, N.; Liu, F.Y.; Ma, S.Q.; An, P.; Yang, D.; Wang, M.Y.; Fan, D.; Yang, Z.; et al. Neuraminidase 1 deficiency attenuates cardiac dysfunction, oxidative stress, fibrosis, inflammatory via AMPK-SIRT3 pathway in diabetic cardiomyopathy mice. Int. J. Biol. Sci. 2022, 18, 826–840.
- Kawaguchi, T.; Takemura, G.; Kanamori, H.; Takeyama, T.; Watanabe, T.; Morishita, K.; Ogino, A.; Tsujimoto, A.; Goto, K.; Maruyama, R.; et al. Prior starvation mitigates acute doxorubicin cardiotoxicity through restoration of autophagy in affected cardiomyocytes. Cardiovasc. Res. 2012, 96, 456–465.
- Sishi, B.J.; Loos, B.; van Rooyen, J.; Engelbrecht, A.M. Autophagy upregulation promotes survival and attenuates doxorubicin-induced cardiotoxicity. Biochem. Pharmacol. 2013, 85, 124–134.
- Govender, J.; Loos, B.; Marais, E.; Engelbrecht, A.M. Mitochondrial catastrophe during doxorubicin-induced cardiotoxicity: A review of the protective role of melatonin. J. Pineal Res. 2014, 57, 367–380.
- Zheng, D.; Zhang, Y.; Zheng, M.; Cao, T.; Wang, G.; Zhang, L.; Ni, R.; Brockman, J.; Zhong, H.; Fan, G.C.; et al. Nicotinamide riboside promotes autolysosome clearance in preventing doxorubicin-induced cardiotoxicity. Clin. Sci. 2019, 133, 1505–1521.
- Sun, Z.; Fang, C.; Xu, S.; Wang, B.; Li, D.; Liu, X.; Mi, Y.; Guo, H.; Jiang, J. SIRT3 attenuates doxorubicin-induced cardiotoxicity by inhibiting NLRP3 inflammasome via autophagy. Biochem. Pharmacol. 2023, 207, 115354.
- Yang, Y.; Li, N.; Chen, T.; Zhang, C.; Li, J.; Liu, L.; Qi, Y.; Zheng, X.; Zhang, C.; Bu, P. Sirt3 promotes sensitivity to sunitinib-induced cardiotoxicity via inhibition of GTSP1/JNK/autophagy pathway in vivo and in vitro. Arch. Toxicol. 2019, 93, 3249–3260.
- He, L.; Wang, J.; Yang, Y.; Zou, P.; Xia, Z.; Li, J. SIRT4 Suppresses Doxorubicin-Induced Cardiotoxicity by Regulating the AKT/mTOR/Autophagy Pathway. Toxicology 2022, 469, 153119.
- Shirakabe, A.; Zhai, P.; Ikeda, Y.; Saito, T.; Maejima, Y.; Hsu, C.P.; Nomura, M.; Egashira, K.; Levine, B.; Sadoshima, J. Drp1-Dependent Mitochondrial Autophagy Plays a Protective Role Against Pressure Overload-Induced Mitochondrial Dysfunction and Heart Failure. Circulation 2016, 133, 1249–1263.
- Zheng, C.B.; Gao, W.C.; Xie, M.; Li, Z.; Ma, X.; Song, W.; Luo, D.; Huang, Y.; Yang, J.; Zhang, P.; et al. Ang II Promotes Cardiac Autophagy and Hypertrophy via Orai1/STIM1. Front. Pharmacol. 2021, 12, 622774.
- Packer, M. Longevity genes, cardiac ageing, and the pathogenesis of cardiomyopathy: Implications for understanding the effects of current and future treatments for heart failure. Eur. Heart J. 2020, 41, 3856–3861.
- Jiang, X.; Zhang, K.; Gao, C.; Ma, W.; Liu, M.; Guo, X.; Bao, G.; Han, B.; Hu, H.; Zhao, Z. Activation of FMS-like tyrosine kinase 3 protects against isoprenaline-induced cardiac hypertrophy by improving autophagy and mitochondrial dynamics. FASEB J. 2022, 36, e22672.
- Wang, H.N.; Li, J.L.; Xu, T.; Yao, H.Q.; Chen, G.H.; Hu, J. Effects of Sirt3-autophagy and resveratrol activation on myocardial hypertrophy and energy metabolism. Mol. Med. Rep. 2020, 22, 1342–1350.
- Li, J.; Chen, T.; Xiao, M.; Li, N.; Wang, S.; Su, H.; Guo, X.; Liu, H.; Yan, F.; Yang, Y.; et al. Mouse Sirt3 promotes autophagy in AngII-induced myocardial hypertrophy through the deacetylation of FoxO1. Oncotarget 2016, 7, 86648–86659.
- Hu, J.; Liu, T.; Fu, F.; Cui, Z.; Lai, Q.; Zhang, Y.; Yu, B.; Liu, F.; Kou, J.; Li, F. Omentin1 ameliorates myocardial ischemia-induced heart failure via SIRT3/FOXO3a-dependent mitochondrial dynamical homeostasis and mitophagy. J. Transl. Med. 2022, 20, 447.
- Gao, J.; Wei, T.; Huang, C.; Sun, M.; Shen, W. Sirtuin 3 governs autophagy-dependent glycolysis during Angiotensin II-induced endothelial-to-mesenchymal transition. FASEB J. 2020, 34, 16645–16661.
- Sundaresan, N.R.; Vasudevan, P.; Zhong, L.; Kim, G.; Samant, S.; Parekh, V.; Pillai, V.B.; Ravindra, P.V.; Gupta, M.; Jeevanandam, V.; et al. The sirtuin SIRT6 blocks IGF-Akt signaling and development of cardiac hypertrophy by targeting c-Jun. Nat. Med. 2012, 18, 1643–1650.
- Lu, J.; Sun, D.; Liu, Z.; Li, M.; Hong, H.; Liu, C.; Gao, S.; Li, H.; Cai, Y.; Chen, S.; et al. SIRT6 suppresses isoproterenol-induced cardiac hypertrophy through activation of autophagy. Transl. Res. 2016, 172, 96–112.e6.
- Wei, T.; Huang, G.; Gao, J.; Huang, C.; Sun, M.; Wu, J.; Bu, J.; Shen, W. Sirtuin 3 Deficiency Accelerates Hypertensive Cardiac Remodeling by Impairing Angiogenesis. J. Am. Heart Assoc. 2017, 6, e006114.
- Zhong, X.L.; Miao, H.J.; Fang, Z.M.; Kuken, B.; Song, H.Y.; Zhong, H.; Lu, Y.; Liu, S.M. The effect of SIRT1 gene polymorphisms on ambulatory blood pressure of hypertensive patients in the Kazakh population. Genet. Test. Mol. Biomark. 2015, 19, 561–565.
- Balarastaghi, S.; Barangi, S.; Hosseinzadeh, H.; Imenshahidi, M.; Moosavi, Z.; Razavi, B.M.; Karimi, G. Melatonin improves arsenic-induced hypertension through the inactivation of the Sirt1/autophagy pathway in rat. Biomed. Pharmacother. 2022, 151, 113135.
- Li, G.; Wang, X.; Yang, H.; Zhang, P.; Wu, F.; Li, Y.; Zhou, Y.; Zhang, X.; Ma, H.; Zhang, W.; et al. α-Linolenic acid but not linolenic acid protects against hypertension: Critical role of SIRT3 and autophagic flux. Cell Death Dis. 2020, 11, 83.
- Sakamoto, J.; Miura, T.; Shimamoto, K.; Horio, Y. Predominant expression of Sir2alpha, an NAD-dependent histone deacetylase, in the embryonic mouse heart and brain. FEBS Lett. 2004, 556, 281–286.
- Lee, D.; Goldberg, A.L. SIRT1 protein, by blocking the activities of transcription factors FoxO1 and FoxO3, inhibits muscle atrophy and promotes muscle growth. J. Biol. Chem. 2013, 288, 30515–30526.
- Li, Z.; Margariti, A.; Wu, Y.; Yang, F.; Hu, J.; Zhang, L.; Chen, T. MicroRNA-199a induces differentiation of induced pluripotent stem cells into endothelial cells by targeting sirtuin 1. Mol. Med. Rep. 2015, 12, 3711–3717.
- Chen, D.; Zheng, K.; Wu, H.; Zhang, X.; Ye, W.; Tan, X.; Xiong, Y. Lin28a attenuates cerebral ischemia/reperfusion injury through regulating Sirt3-induced autophagy. Brain Res. Bull. 2021, 170, 39–48.
- Hao, Y.; Lu, Q.; Yang, G.; Ma, A. Lin28a protects against postinfarction myocardial remodeling and dysfunction through Sirt1 activation and autophagy enhancement. Biochem. Biophys. Res. Commun. 2016, 479, 833–840.
