Eukaryotic cilia are microtubule-based organelles that protrude from the cell surface to fulfill sensory and motility functions. Their basic structure consists of an axoneme templated by a centriole/basal body. Striking differences in ciliary ultra-structures can be found at the ciliary base, the axoneme and the tip, not only throughout the eukaryotic tree of life, but within a single organism. Defects in cilia biogenesis and function are at the origin of human ciliopathies. This structural/functional diversity and its relationship with the etiology of these diseases is poorly understood. Some of the important events in cilia function occur at their distal domain, including cilia assembly/disassembly, IFT (intraflagellar transport) complexes’ remodeling, and signal detection/transduction. How axonemal microtubules end at this domain varies with distinct cilia types, originating different tip architectures. Additionally, they show a high degree of dynamic behavior and are able to respond to different stimuli. The existence of microtubule-capping structures (caps) in certain types of cilia contributes to this diversity.
- cilia
- cilia distal domain
- microtubule-capping structures
- cilia structural diversity
1. Introduction
The Discovery of the Basic Cilia Structure at a Glance
2. CILIA: Variations of a Basic Structure to Fulfill Myriad Functions
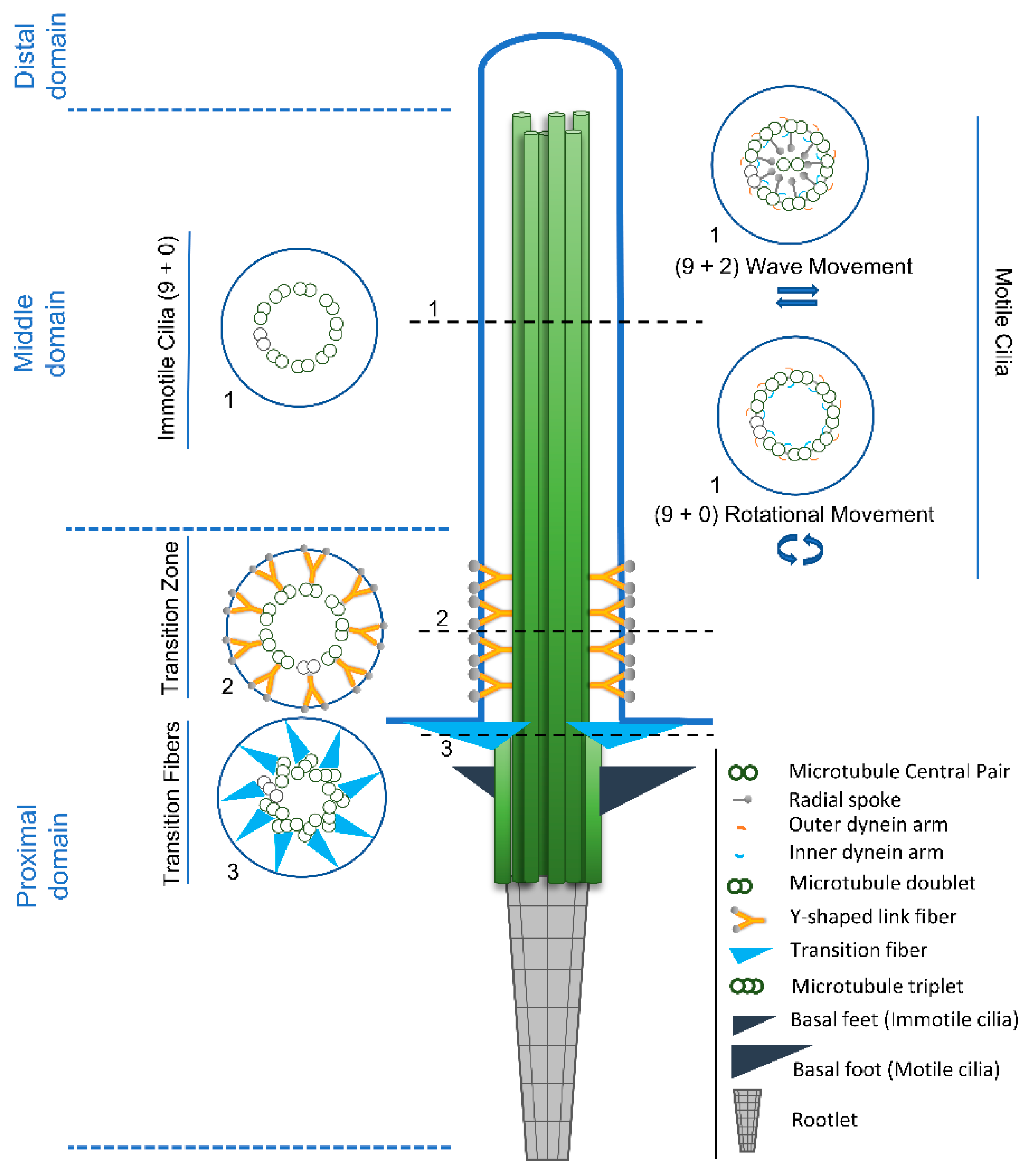
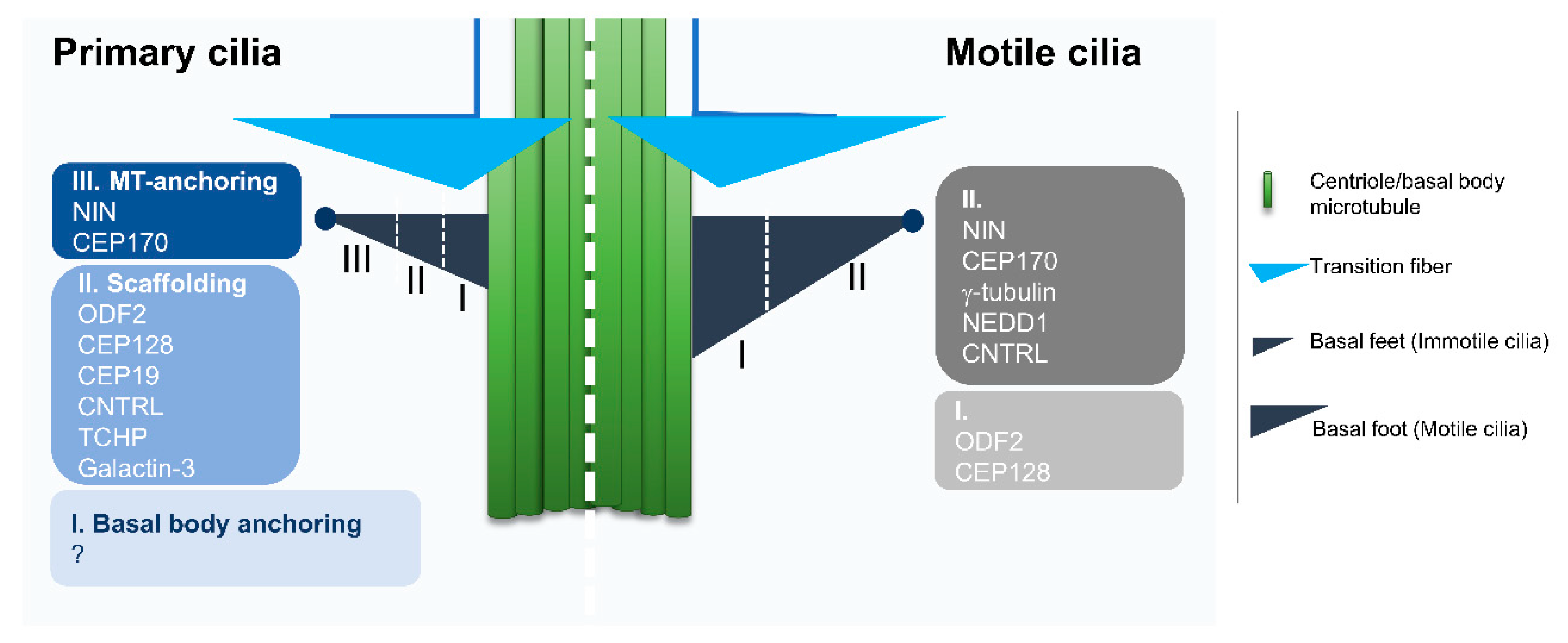
2.1. Diversity Starts at the Base: An Overview
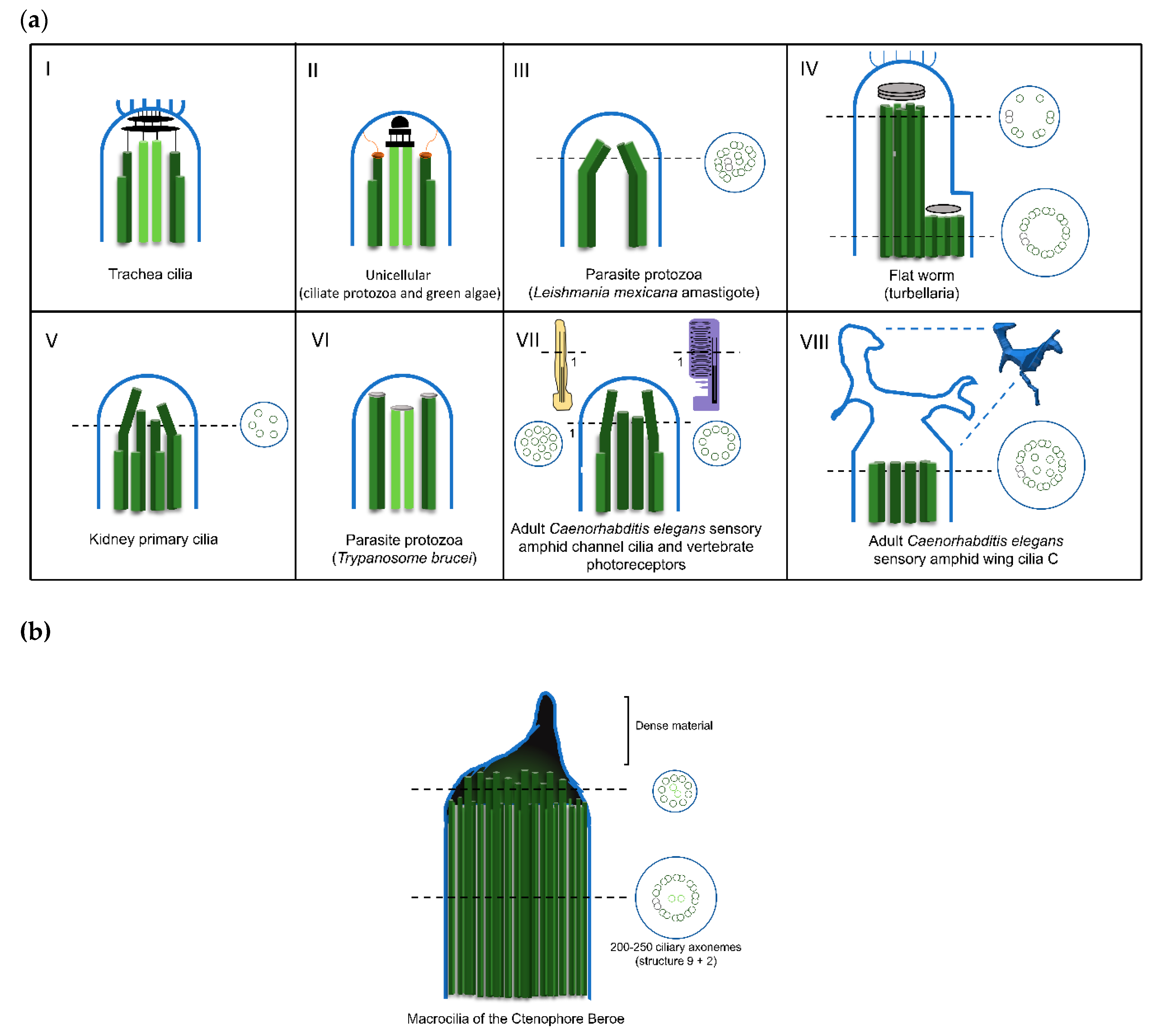
2.2. The Amazing Architecture Diversity of the Cilia Distal Domain: Cilia Tips Segments and Caps
2.3. The Distal Domain of Cilia: Components and Functions
2.4. What Can We Learn about the Functions of the Distal Domain from Proteins Residing There?
This entry is adapted from the peer-reviewed paper 10.3390/cells8020160
References
- Waters, A.M.; Beales, P.L. Ciliopathies: An expanding disease spectrum. Pediatr. Nephrol. 2011, 26, 1039–1056.
- Ostrowski, L.E.; Blackburn, K.; Radde, K.M.; Moyer, M.B.; Schlatzer, D.M.; Moseley, A.; Boucher, R.C. A proteomic analysis of human cilia: Identification of novel components. Mol. Cell Proteom. 2002, 1, 451–465.
- Haimo, L.T.; Rosenbaum, J.L. Cilia, flagella, and microtubules. J. Cell Biol. 1981, 91, 125s–130s.
- Dellinger, O.P. The cilium as a key to the structure of contractile protoplasm. J. Morphol. 1909, 20, 171–210.
- Schmitt, F.O.; Hall, C.E.; Jakus, M.A. The Ultrastructure of Protoplasmic Fibrils. Biol. Symp. 1943, 10, 261–276.
- Harvey, E.B.; Anderson, T.F. The Spermatozoon And Fertilization Membrane Of Arbacia Punctulata As Shown By The Electron Microscope. Biol. Bull. 1943, 85, 151–156.
- Longest, P.M. Structure of the cilia in Ectocarpus mitchellae and Codium decorticatum. J. Elisha Mitchell Sci. Soc. (Chapel Hill, NC) 1946, 62, 249–252.
- Jakus, M.A.; Hall, C.E. Electron microscope observations of the trichocysts and cilia of Paramecium. Biol. Bull. 1946, 91, 141–144.
- Manton, I.; Clarke, B. Electron Microscope Observations on the Spermatozoid of Fucus. Nature 1950, 166, 973–974.
- Fawcett, D.W.; Porter, K.R. A study of the fine structure of ciliated epithelia. J. Morphol. 1954, 94, 221–281.
- Bloodgood, R.A. From Central to Rudimentary to Primary: The History of an Underappreciated Organelle Whose Time Has Come.The Primary Cilium. In Methods in Cell Biology; Academic Press: Cambridge, MA, USA, 2009.
- Heidemann, S.R.; Kirschner, M.W. Aster formation in eggs of Xenopus laevis. Induction by isolated basal bodies. J. Cell Biol. 1975, 67, 105–117.
- Afzelius, B. Electron microscopy of the sperm tail; results obtained with a new fixative. J. Biophys. Biochem. Cytol. 1959, 5, 269–278.
- Gibbons, I.R.; Grimstone, A.V. On flagellar structure in certain flagellates. J. Biophys. Biochem. Cytol. 1960, 7, 697–716.
- Ringo, D.L. Flagellar motion and fine structure of the flagellar apparatus in Chlamydomonas. J. Cell Biol. 1967, 33, 543–571.
- Satir, P. STUDIES ON CILIA: II. Examination of the Distal Region of the Ciliary Shaft and the Role of the Filaments in Motility. J. Cell Biol. 1965, 26, 805–834.
- Bessis, M.; Nomarski, G.; Thiery, J.P.; Breton-Gorius, J. . Rev. Hematol. 13, 249–270.
- Ledbetter, M.C.; Porter, K.R. A “Microtubule” In Plant Cell Fine Structure. J. Cell Biol. 1963, 19, 239–250.
- Slautterback, D.B. Cytoplasmic Microtubules. I. Hydra. J. Cell Biol. 1963, 18, 367–388.
- Watson, M.R.; Hopkins, J.M. Isolated cilia from Tetrahymena pyriformis. Exp. Cell Res. 1962, 28, 280–295.
- Pease, D.C. The Ultrastructure Of Flagellar Fibrils. J. Cell Biol. 1963, 18, 313–326.
- Ledbetter, M.C.; Porter, K.R. Morphology of Microtubules of Plant Cell. Science 1964, 144, 872–874.
- Stephens, R.E.; Renaud, F.L.; Gibbons, I.R.; Stevens, R.E. Guanine nucleotide associated with the protein of the outer fibers of flagella and cilia. Science 1967, 156, 1606–1608.
- Borisy, G.G.; Taylor, E.W. The mechanism of action of colchicine. Binding of colchincine-3H to cellular protein. J. Cell Biol. 1967, 34, 525–533.
- Shelanski, M.L.; Taylor, E.W. Isolation of a protein subunit from microtubules. J. Cell Biol. 1967, 34, 549–554.
- Gibbons, I.R.; Rowe, A.J. Dynein: A Protein with Adenosine Triphosphatase Activity from Cilia. Science 1965, 149, 424–426.
- Cavalier-Smith, T. The phagotrophic origin of eukaryotes and phylogenetic classification of Protozoa. Int. J. Syst. Evol. Microbiol. 2002, 52, 297–354.
- Carvalho-Santos, Z.; Azimzadeh, J.; Pereira-Leal, J.B.; Bettencourt-Dias, M. Tracing the origins of centrioles, cilia, and flagella. J. Cell Biol. 2011, 194, 165–175.
- Mitchell, D.R. The Evolution of Eukaryotic Cilia and Flagella as Motile and Sensory Organelles. In Eukaryotic Membranes and Cytoskeleton; Springer New York: New York, NY, USA, 2007; pp. 130–140.
- Sharma, N.; Kosan, Z.A.; Stallworth, J.E.; Berbari, N.F.; Yoder, B.K. Soluble levels of cytosolic tubulin regulate ciliary length control. Mol. Biol. Cell 2011, 22, 806–816.
- Nicastro, D.; Schwartz, C.; Pierson, J.; Gaudette, R.; Porter, M.E.; McIntosh, J.R. The Molecular Architecture of Axonemes Revealed by Cryoelectron Tomography. Science 2006, 313, 944–948.
- Goetz, S.C.; Anderson, K.V. The primary cilium: A signalling centre during vertebrate development. Nat. Rev. Genet. 2010, 11, 331–344.
- Satir, P.; Christensen, S.T. Overview of Structure and Function of Mammalian Cilia. Annu Rev. Physiol. 2007, 69, 377–400.
- Wheway, G.; Nazlamova, L.; Hancock, J.T. Signaling through the Primary Cilium. Front. Cell Dev. Biol. 2018, 6, 8.
- Bloodgood, R.A. Sensory reception is an attribute of both primary cilia and motile cilia. J. Cell Sci. 2010, 123, 505–509.
- Spirostomum, I.; Author, S.; Jennings, H.S. Studies on Reactions to Stimuli in Unicellular Organisms. III Reactions to Localized Stimuli. Am. Nat. 1899, 33, 373–389.
- Nechipurenko, I.V.; Berciu, C.; Sengupta, P.; Nicastro, D. Centriolar remodeling underlies basal body maturation during ciliogenesis in Caenorhabditis elegans. Elife 2017, 6.
- Jana, S.C.; Mendonça, S.; Machado, P.; Werner, S.; Rocha, J.; Pereira, A.; Maiato, H.; Bettencourt-Dias, M. Differential regulation of transition zone and centriole proteins contributes to ciliary base diversity. Nat. Cell Biol. 2018, 20, 928–941.
- Shahid, U.; Singh, P. Emerging Picture of Deuterosome-Dependent Centriole Amplification in MCCs. Cells 2018, 7, 152.
- Nguyen, Q. P. H., Liu, Z., Albulescu, A., Ouyang, H., Zlock, L., Coyaud, E., Laurent, E., Finkbeiner, W., Moraes, T. J., Raught, B., & Mennella, V. (2020). Comparative Super-Resolution Mapping of Basal Feet Reveals a Modular but Distinct Architecture in Primary and Motile Cilia. Developmental cell, 55(2), 209–223.e7. https://doi.org/10.1016/j.devcel.2020.09.015
- Chen, J.V.; Kao, L.-R.; Jana, S.C.; Sivan-Loukianova, E.; Mendonça, S.; Cabrera, O.A.; Singh, P.; Cabernard, C.; Eberl, D.F.; Bettencourt-Dias, M.; Megraw, T.L.; et al. Rootletin organizes the ciliary rootlet to achieve neuron sensory function in Drosophila. J. Cell Biol. 2015, 211, 435–453.
- Yang, J.; Gao, J.; Adamian, M.; Wen, X.-H.; Pawlyk, B.; Zhang, L.; Sanderson, M.J.; Zuo, J.; Makino, C.L.; Li, T. The Ciliary Rootlet Maintains Long-Term Stability of Sensory Cilia. Mol. Cell Biol. 2005, 25, 4129–4137.
- Mohan, S.; Timbers, T.A.; Kennedy, J.; Blacque, O.E.; Leroux, M.R. Striated Rootlet and Nonfilamentous Forms of Rootletin Maintain Ciliary Function. Curr. Biol. 2013, 23, 2016–2022.
- Gilliam, J.C.; Chang, J.T.; Sandoval, I.M.; Zhang, Y.; Li, T.; Pittler, S.J.; Chiu, W.; Wensel, T.G. Three-Dimensional Architecture of the Rod Sensory Cilium and Its Disruption in Retinal Neurodegeneration. Cell 2012, 151, 1029–1041.
- Dentler, W.L. Cilia and Flagella. In Cytology and Cell Physiology; Elsevier: Amsterdam, The Netherlands, 1987; pp. 391–456.
- Clare, D.K.; Magescas, J.; Piolot, T.; Dumoux, M.; Vesque, C.; Pichard, E.; Dang, T.; Poirier, F. Basal foot MTOC organizes pillar MTs required for coordination of beating cilia. Nat. Commun. 2014, 5, 4888.
- Kunimoto, K.; Yamazaki, Y.; Nishida, T.; Shinohara, K.; Ishikawa, H.; Hasegawa, T.; Okanoue, T.; Hamada, H.; Noda, T.; Tamura, A.; et al. Coordinated Ciliary Beating Requires Odf2-Mediated Polarization of Basal Bodies via Basal Feet. Cell 2012, 148, 189–200.
- Mazo, G.; Soplop, N.; Wang, W.-J.; Uryu, K.; Tsou, M.-F.B. Spatial Control of Primary Ciliogenesis by Subdistal Appendages Alters Sensation-Associated Properties of Cilia. Dev. Cell. 2016, 39, 424–437.
- Reiter, J.F.; Leroux, M.R. Genes and molecular pathways underpinning ciliopathies. Nat. Rev. Mol. Cell Biol. 2017, 18, 533–547.
- Gonçalves, J.; Pelletier, L. The Ciliary Transition Zone: Finding the Pieces and Assembling the Gate. Mol. Cell. 2017, 40, 243–253.
- Gibbons, I.R. The relationship between the fine structure and direction of beat in gill cilia of a lamellibranch mollusc. J. Biophys. Biochem. Cytol. 1961, 11, 179–205.
- Gibbons, I.R. Cilia and flagella of eukaryotes. J. Cell Biol. 1981, 91, 107s–124s.
- Inaba, K. Sperm flagella: Comparative and phylogenetic perspectives of protein components. Mol. Hum. Reprod. 2011, 17, 524–538.
- Kramer-Zucker, A.G.; Olale, F.; Haycraft, C.J.; Yoder, B.K.; Schier, A.F.; Drummond, I.A. Cilia-driven fluid flow in the zebrafish pronephros, brain and Kupffer’s vesicle is required for normal organogenesis. Development 2005, 132, 1907–1921.
- Bird, S.D.; Legge, M.; Walker, R.J. Cultured peritoneal mesothelial cells exhibit apical primary cilia. Cell Biol. Int. 2004, 28, 79–92.
- Moran, D.T.; Rowley, J.C.; Jafek, B.W.; Lovell, M.A. The fine structure of the olfactory mucosa in man. J. Neurocytol. 1982, 11, 721–746.
- Babu, D.; Roy, S. Left-right asymmetry: Cilia stir up new surprises in the node. Open Biol. 2013, 3, 130052.
- Goldstein, S. Motility of the 6+0 flagellum of Lecudina tuzetae. Cell Motil. Cytoskeleton 1982, 2, 369–383.
- Afzelius, B. Cilia-related diseases. J. Pathol. 2004, 204, 470–477.
- Nonaka, S.; Tanaka, Y.; Okada, Y.; Takeda, S.; Harada, A.; Kanai, Y.; Kido, M.; Hirokawa, N. Randomization of left-right asymmetry due to loss of nodal cilia generating leftward flow of extraembryonic fluid in mice lacking KIF3B motor protein. Cell 1998, 95, 829–837.
- Okada, Y.; Takeda, S.; Tanaka, Y.; Belmonte, J.-C.I.; Hirokawa, N. Mechanism of Nodal Flow: A Conserved Symmetry Breaking Event in Left-Right Axis Determination. Cell 2005, 121, 633–644.
- Christensen, S.T.; Guerra, C.; Wada, Y.; Valentin, T.; Angeletti, R.H.; Satir, P.; Hamasaki, T. A Regulatory Light Chain of Ciliary Outer Arm Dynein in Tetrahymena thermophila. J. Biol. Chem. 2001, 276, 20048–20054.
- Habermacher, G.; Sale, W.S. Regulation of flagellar dynein by phosphorylation of a 138-kD inner arm dynein intermediate chain. J. Cell Biol. 1997, 136, 167–176.
- Nicastro, D.; McIntosh, J.R.; Baumeister, W. 3D structure of eukaryotic flagella in a quiescent state revealed by cryo-electron tomography. Proc. Natl. Acad. Sci. USA 2005, 102, 15889–15894.
- Viswanadha, R.; Sale, W.S.; Porter, M.E. Ciliary Motility: Regulation of Axonemal Dynein Motors. Cold Spring Harb. Perspect. Biol. 2017, 9, a018325.
- Inaba, K. Molecular basis of sperm flagellar axonemes: Structural and evolutionary aspects. Ann. N. Y. Acad. Sci. 2007, 1101, 506–526.
- Kollmar, M. Fine-Tuning Motile Cilia and Flagella: Evolution of the Dynein Motor Proteins from Plants to Humans at High Resolution. Mol. Biol. Evol. 2016, 33, 3249–3267.
- Chasey, D. Further observations on the ultrastructure of cilia from Tetrahymena pyriformis. Exp. Cell Res. 1972, 74, 471–479.
- Olson, G.E.; Linck, R.W. Observations of the structural components of flagellar axonemes and central pair microtubules from rat sperm. J. Ultrastruct. Res. 1977, 61, 21–43.
- Hopkins, J.M. Subsidiary Components of the Flagella of Chlamydomonas Reinhardii. J. Cell Sci. 1970, 7.
- Warner, F.D. New observations on flagellar fine structure. The relationship between matrix structure and the microtubule component of the axoneme. J. Cell Biol. 1970, 47, 159–182.
- Barber, C.F.; Heuser, T.; Carbajal-González, B.I.; Botchkarev, V.V.; Nicastro, D. Three-dimensional structure of the radial spokes reveals heterogeneity and interactions with dyneins in Chlamydomonas flagella. Mol. Biol. Cell 2012, 23, 111–120.
- Pigino, G.; Bui, K.H.; Maheshwari, A.; Lupetti, P.; Diener, D.; Ishikawa, T. Cryoelectron tomography of radial spokes in cilia and flagella. J. Cell Biol. 2011, 195, 673–687.
- Pigino, G.; Ishikawa, T. Axonemal radial spokes: 3D structure, function and assembly. Bioarchitecture 2012, 2, 50–58.
- Zhu, X.; Liu, Y.; Yang, P. Radial Spokes—A Snapshot of the Motility Regulation, Assembly, and Evolution of Cilia and Flagella. Cold Spring Harb. Perspect. Biol. 2017, 9, a028126.
- Silva, M.; Morsci, N.; Nguyen, K.C.Q.; Rizvi, A.; Rongo, C.; Hall, D.H.; Barr, M.M. Cell-Specific α-Tubulin Isotype Regulates Ciliary Microtubule Ultrastructure, Intraflagellar Transport, and Extracellular Vesicle Biology. Curr. Biol. 2017, 27, 968–980.
- Phillips, D.M. Insect sperm: Their structure and morphogenesis. J. Cell Biol. 1970, 44, 243–277.
- Dallai, R.; Lupetti, P.; Mencarelli, C. Unusual axonemes of hexapod spermatozoa. Int. Rev. Cytol. 2006, 254, 45–99.
- Fawcett, D.W.; Iton, S. The fine structure of bat spermatozoa. Am. J. Anat. 1965, 116, 567–609.
- Fawcett, D.W. The anatomy of the mammalian spermatozoon with particular reference to the guinea pig. Z Zellforsch Mikrosk Anat. 1965, 67, 279–296.
- Silveira, M.; Porter, K.R. The spermatozoids of Flatworms and their microtubular systems. Protoplasma 1964, 59, 240–265.
- Roth, L.E.; Shigenaka, Y. The structure and formation of cilia and filaments in rumen protozoa. J. Cell Biol. 1964, 20, 249–270.
- Roth, L.E. Aspects of ciliary fine structure in Euplotes patella. J. Biophys. Biochem. Cytol. 1956, 2, 235–240.
- Koch, W.J. Studies of the Motile Cells of Chytrids. I. Electron Microscope Observations of the Flagellum, Blepharoplast and Rhizoplast. Am. J. Bot. 1956, 43, 811.
- Manton, I. Electron microscopical observations on a very small flagellate: The problem of Chromulina pusilla Butcher. J. Mar. Biol. Assoc. UK 1959, 38, 319.
- Tyler, S. An Adhesive Function for Modified Cilia in an Interstitial Turbellarian. Acta Zool. 1973, 54, 139–151.
- Dentler, W.L. Structures linking the tips of ciliary and flagellar microtubules to the membrane. J. Cell Sci. 1980, 42, 207–220.
- Satir, P. Studies on cilia. 3. Further studies on the cilium tip and a "sliding filament" model of ciliary motility. J. Cell Biol. 1968, 39, 77–94.
- Dentler, W.L. Microtubule-Membrane Interactions in Cilia and Flagella. Int. Rev. Cytol. 1981, 72, 1–47.
- Dentler, W.L.; Rosenbaum, J.L. Flagellar elongation and shortening in Chlamydomonas. III. structures attached to the tips of flagellar microtubules and their relationship to the directionality of flagellar microtubule assembly. J. Cell Biol. 1977, 74, 747–759.
- Dentler, W.L. Attachment of the cap to the central microtubules of Tetrahymena cilia. J. Cell Sci. 1984, 66, 167–173.
- Gluenz, E.; Höög, J.L.; Smith, A.E.; Dawe, H.R.; Shaw, M.K.; Gull, K. Beyond 9+0: Noncanonical axoneme structures characterize sensory cilia from protists to humans. FASEB J. 2010, 24, 3117–3121.
- Kuhn, C.; Engleman, W. The structure of the tips of mammalian respiratory cilia. Cell Tissue Res. 1978, 186, 491–498.
- Tamm, S.L.; Tamm, S. Visualization Of Changes In Ciliary Tip Configuration Caused By Sliding Displacement Of Microtubules In Macrocilia Of The Ctenophore Beroe. J. Cell Sci. 1985, 79, 161–179.
- Mukhopadhyay, S.; Lu, Y.; Shaham, S.; Sengupta, P. Sensory Signaling-Dependent Remodeling of Olfactory Cilia Architecture in C. elegans. Dev. Cell. 2008, 14, 762–774.
- Varga, V.; Moreira-Leite, F.; Portman, N.; Gull, K. Protein diversity in discrete structures at the distal tip of the trypanosome flagellum. Proc. Natl. Acad. Sci. USA 2017, 114, E6546–E6555.
- Woolley, D.; Gadelha, C.; Gull, K. Evidence for a sliding-resistance at the tip of the trypanosome flagellum. Cell Motil. Cytoskeleton 2006, 63, 741–746.
- Dirksen, E.R.; Satir, P. Ciliary activity in the mouse oviduct as studied by transmission and scanning electron microscopy. Tissue Cell. 1972, 4, 389–403.
- Jeffery, P.K.; Reid, L. New observations of rat airway epithelium: A quantitative and electron microscopic study. J. Anat. 1975, 120, 295–320.
- Cordier, A.C. Ultrastructure of the cilia of thymic cysts in “nude” mice. Anat Rec. 1975, 181, 227–249.
- Dentler, W.L. Linkages between Microtubules and Membranes in Cilia and Flagella. In Ciliary and Flagellar Membranes; Springer US: Boston, MA, USA, 1990; pp. 31–64.
- Chailley, B.; N’Diaye, A.; Boisvieux-Ulrich, E.; Sandoz, D. Comparative study of the distribution of fuzzy coat, lectin receptors, and intramembrane particles of the ciliary membrane. Eur. J. Cell Biol. 1981, 25, 300–307.
- Tyler, S. Distinctive features of cilia in metazoans and their significance for systematics. Tissue Cell. 1979, 11, 385–400.
- Dentler, W.L.; LeCluyse, E.L. Microtubule capping structures at the tips of tracheal cilia: Evidence for their firm attachment during ciliary bend formation and the restriction of microtubule sliding. Cell Motil. 1982, 2, 549–572.
- LeCluyse, E.L.; Dentler, W.L. Asymmetrical microtubule capping structures in frog palate cilia. J. Ultrastruct. Res. 1984, 86, 75–85.
- Toh, Y.; Yokohari, F. Structure of the antennal chordotonal sensilla of the American cockroach. J. Ultrastruct. Res. 1985, 90, 124–134.
- Owen, G.; McCrae, J.M. Sensory Cell/Gland Cell Complexes Associated with the Pallial Tentacles of the Bivalve Lima hians (Gmelin), with a Note on Specialized Cilia on the Pallial Curtains. Phil. Trans. R. Soc. B 1979, 287, 45–62.
- Pedersen, L.B.; Schrøder, J.M.; Satir, P.; Christensen, S.T. The Ciliary Cytoskeleton. In Comprehensive Physiology; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2012; pp. 779–803.
- Goodenough, U.W. Tipping of flagellar agglutinins by gametes of Chlamydomonas reinhardtii. Cell Motil. Cytoskeleton 1993, 25, 179–189.
- Tuomanen, E. Adjuncts to the therapy of bacterial meningitis. Pediatr. Infect. Dis. J. 1990, 9.
- Anderson, R.G.; Hein, C.E. Distribution of anionic sites on the oviduct ciliary membrane. J. Cell Biol. 1977, 72, 482–492.
- Aiello, E.; Sleigh, M. Ciliary function of the frog oro-pharyngeal epithelium. Cell Tissue Res. 1977, 178, 267–278.
- Johnson, K.A.; Rosenbaum, J.L. Polarity of flagellar assembly in Chlamydomonas. J. Cell Biol. 1992, 119, 1605–1611.
- Witman, G.B. The site of in vivo assembly of flagellar microtubules. Ann. N. Y. Acad. Sci. 1975, 253, 178–191.
- Rosenbaum, J.L.; Carlson, K. Cilia regeneration in Tetrahymena and its inhibition by colchicine. J. Cell Biol. 1969, 40, 415–425.
- Rosenbaum, J.L.; Moulder, J.E.; Ringo, D.L. Flagellar elongation and shortening in Chlamydomonas. The use of cycloheximide and colchicine to study the synthesis and assembly of flagellar proteins. J. Cell Biol. 1969, 41, 600–619.
- Johnson, U.G.; Porter, K.R. Fine structure of cell division in Chlamydomonas reinhardi. Basal bodies and microtubules. J. Cell Biol. 1968, 38, 403–425.
- Marshall, W.F.; Rosenbaum, J.L. Intraflagellar transport balances continuous turnover of outer doublet microtubules. J. Cell Biol. 2001, 155, 405–414.
- Hao, L.; Thein, M.; Brust-Mascher, I.; Civelekoglu-Scholey, G.; Lu, Y.; Acar, S.; Prevo, B.; Shaham, S.; Scholey, J.M. Intraflagellar transport delivers tubulin isotypes to sensory cilium middle and distal segments. Nat. Cell Biol. 2011, 13, 790–798.
- Seixas, C.; Cruto, T.; Tavares, A.; Gaertig, J.; Soares, H. CCTα and CCTδ Chaperonin Subunits Are Essential and Required for Cilia Assembly and Maintenance in Tetrahymena. PLoS ONE 2010, 5, e10704.
- Rich, D.R.; Clark, A.L. Chondrocyte primary cilia shorten in response to osmotic challenge and are sites for endocytosis. Osteoarthr. Cartil. 2012, 20, 923–930.
- Craft, J.M.; Harris, J.A.; Hyman, S.; Kner, P.; Lechtreck, K.F. Tubulin transport by IFT is upregulated during ciliary growth by a cilium-autonomous mechanism. J. Cell Biol. 2015, 208, 223–237.
- Prevo, B.; Mangeol, P.; Oswald, F.; Scholey, J.M.; Peterman, E.J.G. Functional differentiation of cooperating kinesin-2 motors orchestrates cargo import and transport in C. elegans cilia. Nat. Cell Biol. 2015, 17, 1536–1545.
- Kozminski, K.G.; Beech, P.L.; Rosenbaum, J.L. The Chlamydomonas kinesin-like protein FLA10 is involved in motility associated with the flagellar membrane. J. Cell Biol. 1995, 131, 1517–1527.
- Snow, J.J.; Ou, G.; Gunnarson, A.L.; Walker, M.R.S.; Zhou, H.M.; Brust-Mascher, I.; Scholey, J.M. Two anterograde intraflagellar transport motors cooperate to build sensory cilia on C. elegans neurons. Nat. Cell Biol. 2004, 6, 1109–1113.
- Pazour, G.J.; Dickert, B.L.; Witman, G.B. The DHC1b (DHC2) isoform of cytoplasmic dynein is required for flagellar assembly. J. Cell Biol. 1999, 144, 473–481.
- Porter, M.E.; Bower, R.; Knott, J.A.; Byrd, P.; Dentler, W. Cytoplasmic Dynein Heavy Chain 1b Is Required for Flagellar Assembly in Chlamydomonas. Mol. Biol. Cell 1999, 10, 693–712.
- Signor, D.; Wedaman, K.P.; Orozco, J.T.; Dwyer, N.D.; Bargmann, C.I.; Rose, L.S.; Signor, D.; Scholey, J.M. Role of a class DHC1b dynein in retrograde transport of IFT motors and IFT raft particles along cilia, but not dendrites, in chemosensory neurons of living Caenorhabditis elegans. J. Cell Biol. 1999, 147, 519–530.
- Chien, A.; Shih, S.M.; Bower, R.; Tritschler, D.; Porter, M.E.; Yildiz, A. Dynamics of the IFT machinery at the ciliary tip. Elife 2017, 6.
- Pedersen, L.B.; Rosenbaum, J.L. Chapter Two Intraflagellar Transport (IFT). Curr. Top. Dev. Biol. 2008, 85, 23–61.
- Portman, R.W.; LeCluyse, E.L.; Dentler, W.L. Development of microtubule capping structures in ciliated epithelial cells. J. Cell Sci. 1987, 87, 85–94.
- Fok, A.K.; Wang, H.; Katayama, A.; Aihara, M.S.; Allen, R.D. 22S axonemal dynein is preassembled and functional prior to being transported to and attached on the axonemes. Cell Motil. Cytoskeleton 1994, 29, 215–224.
- Qin, H.; Diener, D.R.; Geimer, S.; Cole, D.G.; Rosenbaum, J.L. Intraflagellar transport (IFT) cargo. J. Cell Biol. 2004, 164, 255–266.
- Seixas, C.; Gonçalves, J.; Melo, L.V.; Soares, H. Tetrahymena Cilia Cap is Built in a Multi-step Process: A Study by Atomic Force Microscopy. Protist 2017, 168, 697–717.
- Reynolds, M.J.; Phetruen, T.; Fisher, R.L.; Chen, K.; Pentecost, B.T.; Gomez, G.; Ounjai, P.; Sui, H. The Developmental Process of the Growing Motile Ciliary Tip Region. Sci. Rep. 2018, 8, 7977.
- Dentler, W.L.; LeCluyse, E.L. The effects of structures attached to the tips of tracheal ciliary microtubules on the nucleation of microtubule assembly in vitro. Prog. Clin. Biol. Res. 1982, 80, 13–18.
- Péchart, I.; Kann, M.L.; Levilliers, N.; Bré, M.H.; Fouquet, J.P. Composition and organization of tubulin isoforms reveals a variety of axonemal models. Biol. cell. 1999, 91, 685–697.
- Jensen-Smith, H.C.; Ludueña, R.F.; Hallworth, R. Requirement for the βI and βIV tubulin isotypes in mammalian cilia. Cell Motil. Cytoskeleton 2003, 55, 213–220.
- Bosch Grau, M.; Gonzalez Curto, G.; Rocha, C.; Magiera, M.M.; Marques Sousa, P.; Giordano, T.; Spassky, N.; Janke, C. Tubulin glycylases and glutamylases have distinct functions in stabilization and motility of ependymal cilia. J. Cell Biol. 2013, 202, 441–451.
- Fisch, C.; Dupuis-Williams, P. Ultrastructure of Cilia and Flagella - Back to the Future! Biol. Cell. 2011, 103, 249–270.
- Miller, J.M.; Wang, W.; Balczon, R.; Dentler, W.L. Ciliary Microtubule Capping Structures Contain a Mammalian Kinetochore Antigen. J. Cell Biol. 1990, 110, 703–714.
