Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Chemistry, Organic
Nanofiltration is a type of membrane filtration that employs a semi-permeable membrane. Unlike other types of membrane filtration systems, nanofiltration membranes have pore sizes of less than 10 nm.
- phase inversion
- nanomaterials
- nano-embedded membranes
- metal ions
1. Nanofiltration
Nanofiltration is a type of membrane filtration that employs a semi-permeable membrane. Unlike other types of membrane filtration systems, nanofiltration membranes have pore sizes of less than 10 nm. It lies between ultrafiltration (UF) and reverse osmosis (RO) membranes, which allow the removal of divalent ions such as Mg2+, Ca2+, Pb2+, Co2+, Mn2+ and Zn2+, in addition to dissolved organic matter (DOM) (Figure 3) [23,24,25]. Pressure was forced through a semi-permeable membrane with pores smaller than those of heavy metal ions. The membrane allows water molecules to move while capturing heavy metal ions and other contaminants [26,27].
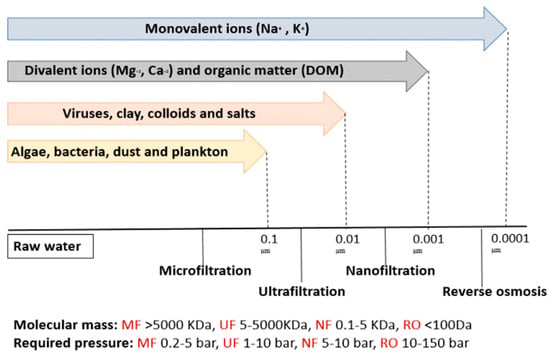
Figure 3. The representation of membrane types utilized in water treatment.
Fabrication techniques for nanofiltration (NF) involve two main processes: interfacial polymerization (IP) and phase inversion (PI). Notably, the aqueous and organic phases are the main components in the formation of the NF membranes through IP. Hence, some NF membranes may be used as initiators or catalysts. At the interface, polymerization occurs once the solutions are in contact. The monomers of the two solutions are divided, and the polymerization occurs through the film. This results in the formation of a thin film, and the thickness of the polymer film increases with time [28]. This process may include the reaction between a ligand with a vacant orbital and a metal ion with an extra lone pair to form a coordinate bond. Metal–ligand complexes in which the polymer film on the membrane surface are originally functionalized with ligands that possess a high affinity for heavy metal ions, such as carboxylic acid or amine groups. However, this model is uncommon in the fabrication of nanofiltration membranes [29]. The popular compounds used in the formation of metal-ligand complexes are metal–organic frameworks (MOF).
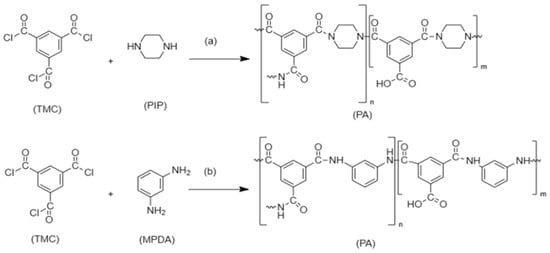
Seah et al. [30] fabricated a nanofiltration membrane by reacting trimesoyl chloride (TMC) monomer ‘organic phase’ with piperazine (PIP) monomer ‘aqueous phase’. The IP then takes place to form the polyamide TFC, as demonstrated in Figure 4a. Another example is the interfacial polymerization between trimesoyl chloride (TMC) and m-phenylenediamine (MPDA) to form TFC polyamide, as shown in Figure 4b, which achieves great removal of heavy metals with the assistance of polyethersuflone (PES) as a support layer. The reaction took place between the chloride atom in (TMC) which started the reaction with the hydrogen atom in (MDPA) to form an amide bond [31].
Another process is phase inversion by the introduction of non-solvent ‘water’ to form non-solvent-induced phase separation or, in some cases, is named ‘immersion precipitation’. This process is illustrated in Figure 5. where a polymer or polymer combination is dissolved in at least one solvent to form a dope solution. The dope solution is subsequently poured as a liquid layer onto a substrate, a glass plate, or a polymeric substrate [33]. It includes the creation of two phases by performing the exchange of a non-solvent from a coagulation bath, usually water, for the solvent from the polymer solution. A solution with a high polymer content is responsible for the production of the membrane matrix. The second phase includes only a very small amount of polymer [34]. After that, the membrane was dried in an oven or at room temperature and then ready to enter the nanofiltration system.
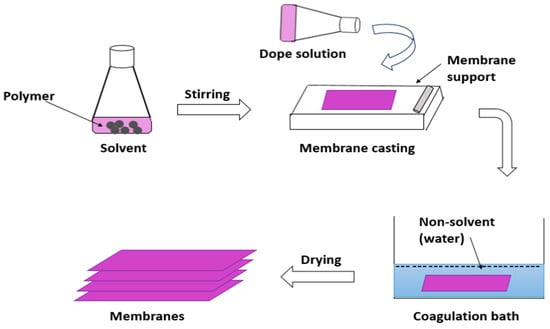
Figure 5. Immersion precipitation process in which the polymeric matrix dissolved with solvents to form membranes.
Fabrication of sheet membranes in NF utilizing chitosan/polyvinyl alcohol and montmorillonite clay was followed by a non-solvent-induced phase inversion technique and exhibited better performance in the removal of chromium [35]. The (NF) membrane was fabricated with the help of a Spinneret incorporating a dry-jet wet. The introduction of polybenzimidazole and polyethersulfone/polyvinylpyrrolidone (PBI-PES/PVP) dopes was introduced through a phase inversion process [36]. Positively charged, highly permeable nanofiltration membranes were fabricated by the phase inversion method using the poly(acid–base) complexation effect for heavy metal elimination [37].
The membrane structure is composed of three layers (1) a nonwoven polymeric support ‘’polyethylene terephthalate’’, (2) a microporous polymeric support ‘’polysulfone’’, and (3) a thin separation layer consisting of cross-linked ‘’polyamide’’ [38]. Polyaniline nanoparticles were added to polysulfone/chitosan by phase inversion to form a nanofiltration membrane. The water flux recorded was 8.04 L m−2 h−1. The rejection of copper was >86% [39]. The engagement of polyethersulfone (PES) by 0.5 wt% to graphene oxide (GO) via phase inversion. This resulted in the formation of sulfonated graphene oxide (sGO-0.5) nanoparticles, which resulted in a dense membrane and formed a finger-like structure with mass growth. It exhibited the best performance in comparison with bare polyethersulfone (PES) and graphene oxide (GO). The rejection of heavy metals was the best in sulfonated graphene oxide (sGO-0.5), in the order of Cr > Cd > Cu > Ni. Additionally, sGO-0.5 exhibited the best performance in terms of resistance to fouling [40].
Ongoing research aims to introduce new fabrication methods for the removal of heavy metals by nanofiltration. Among various methods to remove contaminants from discharged land streams, nanofiltration is favoured over other processes because of the innovation of nanoparticles. Nanoparticles produce membranes with high simplicity, manufacturing scalability, and energy efficiency [41]. Nanofillers are forms of nanoparticles that contribute to membrane filtration by enhancing the thermal, chemical, and mechanical properties of membranes as well as membrane separation characteristics. The conduction of nanofillers to the membrane surface facilitates the compatibility between the fillers and the polymeric matrices, which mainly includes the surface modification of nanomaterials with reactive moieties [42].
2. Mechanism of Removal of Heavy Metals by NF
Nanofiltration membranes use membrane micropores to filter and employ the selective permeability of membranes to separate particulates from wastewater. The nanofiltration membrane acted as a barrier in the separating feed solution. These barriers control the passage of species. Membrane pore size has an effective selectivity for heavy metals. The smaller the membrane pore size, the higher the separation of heavy metals. Steric hindrance is responsible for the rejection of ions with diameters larger than the pore size of the membrane [64]. Size exclusion and the Donnan effect are considered important methods for measuring membrane efficiency during separation [65]. Size exclusion occurs when the ions of feed solution at different sizes are introduced onto the nanofiltration membrane, and the membrane starts to select specific ions to pass through it [66]. The Donnan effect is another mechanism of separation with the assistance of a semipermeable membrane, in which selective ions assemble on one side of the barrier and undesired ions are on the other side [67]. In nanofiltration techniques, the Donnan effect is widely used and effective for ion separation [68].
Shao et al. [69] formed a PEI/GO (polyethyleneimine/graphene oxide) thin film nanocomposite and used size exclusion and the Donnan effect to measure the selectivity of ions. PEI/GO. The membrane exhibited a positive surface charge and sharp repulsion with monovalent and divalent ions. However, the membrane possesses a full-fed attraction to negatively charged ions. Rejection mechanisms include (1) the formation of hydrogen bonds between water and membrane molecules in which heavy metals are transported. (2) Electrostatic repulsion is another rejection mechanism towing to the difference in the dielectric constant between the membrane and solution. (3) The charged capillary mechanism is the electric double layer in which ions of the same charge of the membrane are attracted while different charges are rejected. (4) Diffusion mechanism by the desolvation of the solute and solvent, diffusion of the solvent, and diffusion through the membrane [70].
This entry is adapted from the peer-reviewed paper 10.3390/membranes13090789
This entry is offline, you can click here to edit this entry!
