MEA-based electrical stimulation activates retinal ganglion cells (RGCs), producing phosphene-based artificial vision. However, the lack of spatial selectivity in MEA stimulation leads to coarse and unreliable phosphenes. To improve selectivity, return electrodes and stimulation parameter manipulation are proposed. Patient experiences with retinal prostheses differ, resembling a "light show" rather than natural vision. The shape and strength of the electric field impact visual perception, and lateral spreading reduces selectivity. Epiretinal devices face bundle activation challenges, while subretinal prostheses exhibit variable phosphene shapes. Enhancing spatial resolution requires reliable isolated points of light. Return electrodes and stimulation parameter control are employed to achieve selectivity. Bidirectional/closed-loop systems record cell responses and optimize stimulation, while computational models aid optimization and understanding of retinal activity. These advancements aim to enhance retinal prostheses, providing a more natural and reliable visual experience.
- Spatial Selectivity
- Electrical stimulation
- Microelectrode arrays (MEAs)
- Retinal ganglion cells (RGCs)
- Artificial vision
- Retinal prostheses
- Ophthalmology
1. Spatial Selectivity
Electrical stimulation through microelectrode arrays (MEAs) enables retinal ganglion cells (RGCs) to depolarize and transmit visual signals to the brain[1]. This stimulation produces a phosphene, which is the fundamental unit of artificial vision and subjectively perceived by recipients of retinal prostheses. In an ethnographic study, patients described their visual experience with retinal prostheses as distinctly different from natural vision, resembling a "light show"[2]. The dissimilarities between artificial and natural vision can be attributed to the coarse pattern of retinal activation caused by MEA stimulation, lacking coordination among neighboring cells[1]. The concern lies not only in the deviation from natural vision but also in the unreliable and irregularly shaped phosphenes resulting from such unselective activation[3]. The shape and strength of the electric field produced by a stimulating electrode directly impact the visual percept. Lateral spreading of the electric field can activate multiple retinal cells simultaneously, leading to a loss of spatial selectivity (Figure 1)[4].
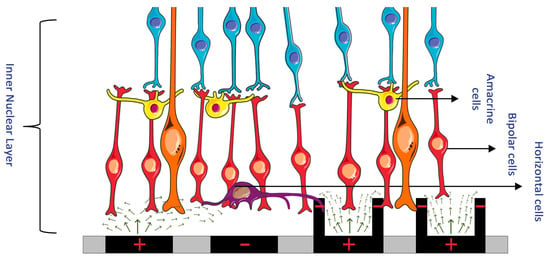
Figure 1. (Left) Electric field of a bipolar configuration causing lateral spread and unselectively stimulating many retinal cells. (Right) A 3D geometry electrode with circumferential returns generates locally confined electric fields, reducing electrode cross-talk and permitting more selective activation of retinal cells. (Figure 1 was partly generated using Servier Medical Art, provided by Servier, licensed under a Creative Commons Attribution 3.0 Unported License.)
In epiretinal devices, MEAs unintentionally activate bundles of RGC axons in the nerve fiber layer, as these axons lie closer to the electrodes than the intended targets, the RGC somas[5]. Consequently, elongated or arc-like phosphenes negatively affect visual quality[6]. Subretinal prostheses, though less prone to interference from RGC axons, can still exhibit highly variable phosphene shapes across electrodes and between subjects due to unwanted excitation of various cell types, including amacrine cells[7][8][9].
To enhance spatial resolution and vision, it is necessary for individual electrodes of retinal prostheses to reliably produce isolated points of light that the brain can assemble into objects, akin to an electronic scoreboard[10]. However, achieving this level of precision and selectivity in retinal stimulation remains a challenge. Various engineering approaches have been proposed to address this issue, with two predominant methods standing out. The first involves the use of return electrodes to localize the electric field, enabling selective stimulation of specific cells. The second method involves manipulating electric stimulation parameters, such as amplitude and frequency, to selectively target cells that respond to specific parameters while disregarding others. These strategies aim to overcome the limitations of unselective and imprecise retinal stimulation, paving the way for substantial improvements in spatial resolution and overall visual perception.
Overall, advancements in retinal prostheses necessitate addressing engineering design challenges to enhance the outcomes of electrical stimulation. By refining stimulation techniques and achieving spatial selectivity, researchers aim to provide recipients with a more natural and reliable visual experience.
2. Return Electrodes for Electric Field Localization and Current Steering
Retinal cell stimulation primarily relies on depolarizing cells within an electric field, rather than direct current injection[4]. Charge accumulation at synaptic terminals leads to membrane depolarization surpassing the threshold for firing action potentials. Controlling the electric field shape is crucial for selective stimulation of target cells. Researchers incorporated adjacent return or grounding electrodes into MEAs to achieve such control. Return electrodes serve as electrical counterparts to active electrodes, limiting current spread and producing a well-controlled field. Various return electrode configurations have been explored: monopolar, bipolar, tripolar, and hexapolar setups[4]. Figure 2 illustrates these configurations. Monopolar stimulation utilizes a single electrode; bipolar stimulation involves an active electrode with an adjacent return electrode, enabling current steering and localization. In the tripolar configuration, two electrodes on opposite sides of the stimulating electrode function as return electrodes. The hexapolar configuration features a centrally located active electrode surrounded by six return electrodes forming a "guard"[4]. Recent investigations have proposed more complex configurations, such as a 3D concentric bipolar electrode for highly focused stimulation [49]. Another method involves dynamic electric field confinement by using designated active pixels as transient returns[8]. Computational implant simulators have also been developed to model electric fields generated by MEAs, aiding in optimization[11]. These advancements, including the ideation of return electrodes and computational modeling, enable confined electric fields and selective targeting of desired cells[1].
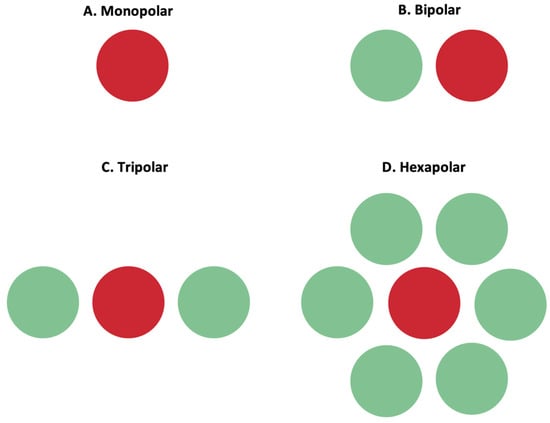
Figure 2. Active (red) and return (green) electrodes arranged in different configurations. (A) Monopolar configuration; (B) bipolar configuration; (C) tripolar configuration; (D) hexapolar configuration. These configurations impact the shape of the electric field, and the return electrode is used to limit current spread and produce a well-controlled, directed electric field.
However, current steering alone does not address bundle activation challenges in the nerve fiber layer, particularly in epiretinal electrodes where proximity to the nerve fiber layer poses difficulties. Selective activation of somas requires an alternative cell stimulation method[4]. Electric stimulation parameter control has been proposed as a solution, which will be discussed in the following section.
3. Electric Stimulation Parameters for Selective Cell Activation and Chromatic Vision
An alternative approach to selectively activate retinal cells involves manipulating the electric impulses sent by the electrodes[4]. Instead of relying on electric field confinement, this strategy focuses on optimizing the pattern of electrical stimulation, including pulse amplitude and frequency, to target specific cells while leaving others inactive. This approach offers several advantages over electric field confinement.
Firstly, there are over 40 types of retinal ganglion cells (RGCs), each responsible for different aspects of visual information processing[12]. Selectively stimulating each type of RGC is desirable for the full restoration of vision[10]. Customized electrical stimuli from independent electrodes can be used to target different cell types, accommodating variations in disease genotypes[13]. This individualized approach is crucial for tailoring the prosthetic vision experience to specific patients.
Secondly, frequency-modulated electrical stimulation has shown promise for restoring chromatic vision in blind patients[14]. Studies have demonstrated that manipulating stimulation parameters can evoke color perception, allowing blind individuals to perceive multiple colors[15][16][17][18]. This suggests that selective activation of specific cell types using appropriate stimulation parameters can provide rudimentary color vision to blind patients.
Combining the selective activation methods of current steering and electric stimulation offers even greater selectivity and control[19][20]. Current steering allows for precise targeting of cells and minimizes cross-talk. By modulating the current ratio between adjacent electrodes, biased electric fields can be created, shifting the virtual electrode towards the desired target[20]. This method enhances target cell selectivity while reducing the activation of neighboring cells. Experimental results closely matched the predicted spatial pattern, achieving a 90% target cell activation probability with only an 11% probability of activating neighboring cells[20].
In summary, manipulating electric impulses sent by the electrodes provides an alternative approach to selectively activate retinal cells. This strategy allows for targeted activation of specific cell types and accommodates variations in disease genotypes. Additionally, frequency-modulated electrical stimulation can restore rudimentary color vision in blind patients. Current steering, in combination with electric stimulation, offers greater selectivity and control, minimizing cross-talk and enhancing target cell activation. These approaches hold promise for advancing retinal prostheses and improving the quality of vision restoration in individuals with retinal diseases.
4. Bidirectional/Closed-Loop Retinal Prostheses
Recent advancements in retinal prostheses include the development of bidirectional and closed-loop systems, which can both send electric impulses to retinal cells and record their responses[21]. This area of research has gained increased attention due to several reasons.
Firstly, the neural plasticity of the retina and the dynamic changes in its electrophysiologic properties over the course of disease necessitate iterative manipulation of electrical patterns for optimal outcomes[22][23][24]. The progressive degeneration of the retina can result in increased electrical thresholds required by subretinal and epiretinal prostheses[24].
Secondly, bidirectional systems offer fully representative, personalized, and iterative stimulation strategies. Ex vivo experiments lack the metabolic environment of the eye, which influences the true electrophysiologic properties of the retina[21]. Bidirectional systems provide a more comprehensive understanding of retinal responses to stimulation in vivo, filling gaps in knowledge obtained from ex vivo testing.
Furthermore, bidirectional systems are crucial due to the variable electrode-retina distance upon implantation[25]. This variability affects stimulation efficacy and the resulting phosphenes[7]. Closed-loop systems that guide stimulation based on evoked cell responses can significantly improve patient outcomes by enabling post-implantation customization.
Closed-loop systems also address biological challenges. For example, some studies have used bidirectional epiretinal prostheses to detect axon bundle activation or develop real-time optimization methods for focal responses[3][26].
The incorporation of bidirectional closed-loop systems requires understanding how recorded cell responses inform feedback for targeted stimulation strategies[1]. Computational models have emerged to analyze the electrical activity of the retina, the impact of stimulation parameters on different cell types, and varying levels of degeneration[27][28][29][30][31]. These models aid researchers and engineers in optimizing retinal prostheses.
In summary, bidirectional and closed-loop systems in retinal prostheses offer the ability to both stimulate retinal cells and record their responses. The dynamic nature of the retina's electrophysiologic properties, the need for personalized stimulation strategies, and the variability in electrode-retina distance drive the development of these systems. Computational models support the understanding of retinal activity and the effects of stimulation parameters. These advancements hold promise for improving the efficacy and customization of retinal prostheses.
References
- Fan, V.H.; Grosberg, L.E.; Madugula, S.S.; Hottowy, P.; Dabrowski, W.; Sher, A.; Litke, A.M.; Chichilnisky, E.J. Epiretinal Stimulation with Local Returns Enhances Selectivity at Cellular Resolution. J. Neural Eng. 2019, 16, 025001.
- Erickson-Davis, C.; Korzybska, H. What Do Blind People “See” with Retinal Prostheses? Observations and Qualitative Reports of Epiretinal Implant Users. PLoS ONE 2021, 16, e0229189.
- Tandon, P.; Bhaskhar, N.; Shah, N.; Madugula, S.; Grosberg, L.; Fan, V.H.; Hottowy, P.; Sher, A.; Litke, A.M.; Chichilnisky, E.J.; et al. Automatic Identification of Axon Bundle Activation for Epiretinal Prosthesis. IEEE Trans. Neural Syst. Rehabil. Eng. 2021, 29, 2496–2502.
- Tong, W.; Meffin, H.; Garrett, D.J.; Ibbotson, M.R. Stimulation Strategies for Improving the Resolution of Retinal Prostheses. Front. Neurosci. 2020, 14, 262.
- Grosberg, L.E.; Ganesan, K.; Goetz, G.A.; Madugula, S.S.; Bhaskhar, N.; Fan, V.; Li, P.; Hottowy, P.; Dabrowski, W.; Sher, A.; et al. Activation of Ganglion Cells and Axon Bundles Using Epiretinal Electrical Stimulation. J. Neurophysiol. 2017, 118, 1457–1471.
- Weitz, A.C.; Nanduri, D.; Behrend, M.R.; Gonzalez-Calle, A.; Greenberg, R.J.; Humayun, M.S.; Chow, R.H.; Weiland, J.D. Improving the Spatial Resolution of Epiretinal Implants by Increasing Stimulus Pulse Duration. Sci. Transl. Med. 2015, 7, 318ra203.
- Ghaffari, D.H.; Chang, Y.-C.; Mirzakhalili, E.; Weiland, J.D. Closed-Loop Optimization of Retinal Ganglion Cell Responses to Epiretinal Stimulation: A Computational Study. In Proceedings of the 2021 10th International IEEE/EMBS Conference on Neural Engineering (NER), Virtual Event, Italy, 4–6 May 2021; pp. 597–600.
- Wang, B.-Y.; Chen, Z.C.; Bhuckory, M.; Huang, T.; Shin, A.; Zuckerman, V.; Ho, E.; Rosenfeld, E.; Galambos, L.; Kamins, T.; et al. Electronic Photoreceptors Enable Prosthetic Visual Acuity Matching the Natural Resolution in Rats. Nat. Commun. 2022, 13, 6627.
- Madugula, S.S.; Gogliettino, A.R.; Zaidi, M.; Aggarwal, G.; Kling, A.; Shah, N.P.; Vilkhu, R.; Hays, M.R.; Nguyen, H.; Fan, V.; et al. Focal Electrical Stimulation of Human Retinal Ganglion Cells for Vision Restoration. J. Neural Eng. 2022, 19, 066040.
- Yunzab, M.; Soto-Breceda, A.; Maturana, M.; Kirkby, S.; Slattery, M.; Newgreen, A.; Meffin, H.; Kameneva, T.; Burkitt, A.N.; Ibbotson, M.; et al. Preferential Modulation of Individual Retinal Ganglion Cells by Electrical Stimulation. J. Neural Eng. 2022, 19, 045003.
- Chen, Z.C.; Wang, B.-Y.; Goldstein, A.K.; Butt, E.; Mathieson, K.; Palanker, D. Photovoltaic Implant Simulator Reveals Resolution Limits in Subretinal Prosthesis. J. Neural Eng. 2022, 19, 055008.
- Goetz, J.; Jessen, Z.F.; Jacobi, A.; Mani, A.; Cooler, S.; Greer, D.; Kadri, S.; Segal, J.; Shekhar, K.; Sanes, J.R.; et al. Unified Classification of Mouse Retinal Ganglion Cells Using Function, Morphology, and Gene Expression. Cell Rep. 2022, 40, 111040.
- Roh, H.; Otgondemberel, Y.; Eom, J.; Kim, D.; Im, M. Electrically-Evoked Responses for Retinal Prostheses Are Differentially Altered Depending on Ganglion Cell Types in Outer Retinal Neurodegeneration Caused by Crb1 Gene Mutation. Front. Cell. Neurosci. 2023, 17, 1115703.
- Yue, L.; Castillo, J.; Gonzalez, A.C.; Neitz, J.; Humayun, M.S. Restoring Color Perception to the Blind: An Electrical Stimulation Strategy of Retina in Patients with End-Stage Retinitis Pigmentosa. Ophthalmology 2021, 128, 453–462.
- Stanga, P.E.; Hafezi, F.; Sahel, J.A.; daCruz, L.; Merlini, F.; Coley, B.; Greenberg, R.J.; Argus II Study Group. Patients Blinded By Outer Retinal Dystrophies Are Able To Perceive Color Using The ArgusTm II Retinal Prosthesis System. Investig. Ophthalmol. Vis. Sci. 2011, 52, 4949.
- Stanga, P.E.; Sahel, J.A., Jr.; daCruz, L.; Hafezi, F.; Merlini, F.; Coley, B.; Greenberg, R.J.; Argus II Study Group. Patients Blinded by Outer Retinal Dystrophies Are Able to Perceive Simultaneous Colors Using the Argus® II Retinal Prosthesis System. Investig. Ophthalmol. Vis. Sci. 2012, 53, 6952.
- Towle, V.L.; Pham, T.; McCaffrey, M.; Allen, D.; Troyk, P.R. Toward the Development of a Color Visual Prosthesis. J. Neural Eng. 2021, 18, 023001.
- Paknahad, J.; Loizos, K.; Yue, L.; Humayun, M.S.; Lazzi, G. Color and Cellular Selectivity of Retinal Ganglion Cell Subtypes through Frequency Modulation of Electrical Stimulation. Sci. Rep. 2021, 11, 5177.
- Shim, S.; Eom, K.; Jeong, J.; Kim, S.J. Retinal Prosthetic Approaches to Enhance Visual Perception for Blind Patients. Micromachines 2020, 11, 535.
- Jepson, L.H.; Hottowy, P.; Mathieson, K.; Gunning, D.E.; Dąbrowski, W.; Litke, A.M.; Chichilnisky, E.J. Spatially Patterned Electrical Stimulation to Enhance Resolution of Retinal Prostheses. J. Neurosci. 2014, 34, 4871–4881.
- Vėbraitė, I.; Hanein, Y. In the Eye of the Storm: Bi-Directional Electrophysiological Investigation of the Intact Retina. Front. Neurosci. 2022, 16, 829323.
- Caravaca-Rodriguez, D.; Gaytan, S.P.; Suaning, G.J.; Barriga-Rivera, A. Implications of Neural Plasticity in Retinal Prosthesis. Investig. Ophthalmol. Vis. Sci. 2022, 63, 11.
- Kang, H.; Abbasi, W.H.; Kim, S.-W.; Kim, J. Fully Integrated Light-Sensing Stimulator Design for Subretinal Implants. Sensors 2019, 19, 536.
- Xu, A.; Beyeler, M. Retinal Ganglion Cells Undergo Cell Type-specific Functional Changes in a Biophysically Detailed Model of Retinal Degeneration. BioRxiv 2023.
- Avraham, D.; Yitzhaky, Y. Simulating the Perceptual Effects of Electrode–Retina Distance in Prosthetic Vision. J. Neural Eng. 2022, 19, 035001.
- Haji Ghaffari, D.; Akwaboah, A.D.; Mirzakhalili, E.; Weiland, J.D. Real-Time Optimization of Retinal Ganglion Cell Spatial Activity in Response to Epiretinal Stimulation. IEEE Trans. Neural Syst. Rehabil. Eng. 2021, 29, 2733–2741.
- Ly, K.; Guo, T.; Tsai, D.; Muralidharan, M.; Shivdasani, M.N.; Lovell, N.H.; Dokos, S. Simulating the Impact of Photoreceptor Loss and Inner Retinal Network Changes on Electrical Activity of the Retina. J. Neural Eng. 2022, 19, 065002.
- Paknahad, J.; Kosta, P.; Bouteiller, J.-M.C.; Humayun, M.S.; Lazzi, G. Mechanisms Underlying Activation of Retinal Bipolar Cells through Targeted Electrical Stimulation: A Computational Study. J. Neural Eng. 2021, 18, 066034.
- Iseri, E.; Kosta, P.; Paknahad, J.; Bouteiller, J.-M.C.; Lazzi, G. A Computational Model Simulates Light-Evoked Responses in the Retinal Cone Pathway. In Proceedings of the 2021 43rd Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC), Guadalajara, Mexico, 1–5 November 2021; pp. 4482–4486.
- Italiano, M.L.; Guo, T.; Lovell, N.H.; Tsai, D. Improving the Spatial Resolution of Artificial Vision Using Midget Retinal Ganglion Cell Populations Modeled at the Human Fovea. J. Neural Eng. 2022, 19, 035002.
- Relic, L.; Zhang, B.; Tuan, Y.-L.; Beyeler, M. Deep Learning-Based Perceptual Stimulus Encoder for Bionic Vision. In Proceedings of the Augmented Humans International Conference, Chiba, Japan, 13–15 March 2022.
