Antibiotic and multi-drug resistant (MDR) Salmonella poses a significant threat to public health due to its ability to colonize animals (cold and warm-blooded) and contaminate freshwater supplies. Monitoring antibiotic resistant Salmonella is traditionally costly, involving the application of phenotypic and genotypic tests over several days.
- multi-drug resistant
- Salmonella
- detection
- phenotypic
- genotypic
1. Introduction
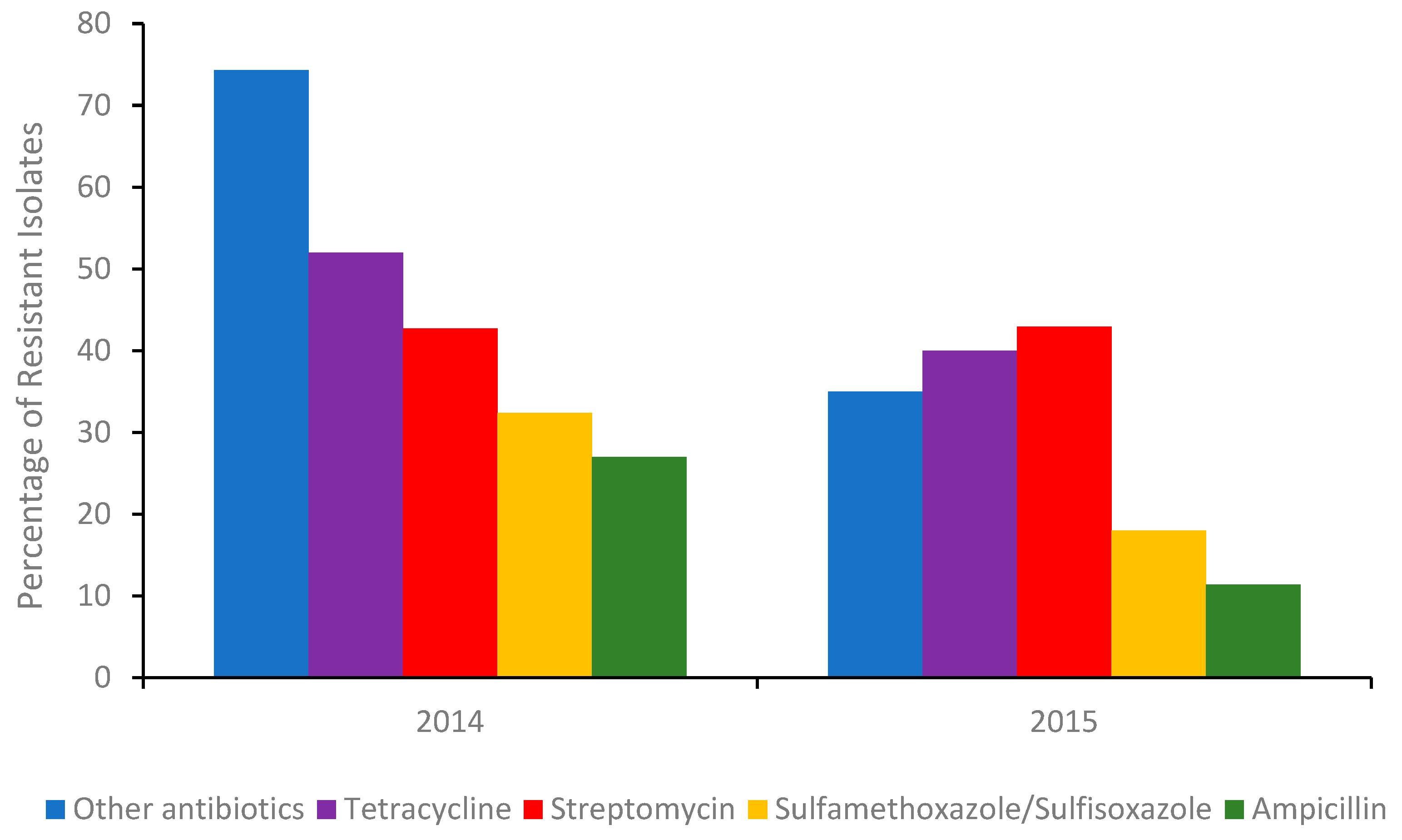
2. Conventional Isolation, Enrichment and Detection Methods
3. Automated Whole Genome Sequencing
4. Automated Phenotypic Testing
4.1. Manual and Semi-Automated Antimicrobial Susceptibility Test (AST)
4.2. Detection of Intracellular Resistance Salmonella Using Flow Cytometry
5. Emerging Biosensors
|
Salmonella Serotypes |
Sensing Method |
Sample Matrix |
Analysis Time (min) |
Detection Limit (CFU/mL) |
Reference |
|---|---|---|---|---|---|
|
S. Typhimurium DT104 |
SERS |
Assay media |
30 |
105 |
[53] |
|
S. Typhimurium DT104 |
SERS |
Assay media |
15 |
105 |
[55] |
|
S. Typhimurium |
SERS |
H2O & milk |
120 |
20 |
[54] |
|
S. enterica |
Raman Spectroscopy |
Urine |
150 |
n/a |
[56] |
|
S. Enteritidis, |
Fluorescence |
Water, milk, and beef |
30 |
2.0 × 102 |
[57] |
|
S. Typhimurium DT104 and S. Typhi |
SERS |
Assay media |
120 |
100 |
[58] |
This entry is adapted from the peer-reviewed paper 10.3390/ijms22073499
References
- Grimont, P.A.; Weill, F.X. Antigenic. Formulae of Salmonella Serovars, 9th ed.; WHO Collaborating Centre for Reference and Research on Salmonella: Geneva, Switzerland, 2007.
- Bugarel, M.; Granier, S.A.; Weill, F.X.; Fach, P.; Brisabois, A. A multiplex real-time PCR assay targeting virulence and resistance genes in Salmonella enterica serotype Typhimurium. BMC Microbiol. 2011, 11, 151.
- WHO. WHO Estimates of the Global Burden of Foodborne Diseases: Foodborne Disease Burden Epidemiology Reference Group 2007–2015; WHO: Geneva, Switzerland, 2016.
- Lee, K.M.; Runyon, M.; Herrman, T.J.; Phillips, R.; Hsieh, J. Review of Salmonella detection and identification methods: Aspects of rapid emergency response and food safety. Food Control. 2015, 47, 264–276.
- Stephen Inbaraj, B.; Chen, B.H. Nanomaterial-based sensors for detection of foodborne bacterial pathogens and toxins as well as pork adulteration in meat products. J. Food. Drug. Anal. 2016, 24, 15–28.
- Farooq, U.; Yang, Q.; Ullah, M.W.; Wang, S. Bacterial biosensing: Recent advances in phage-based bioassays and biosensors. Biosens. Bioelectron. 2018, 118, 204–216.
- Chong, A.; Lee, S.; Yang, Y.A.; Song, J. The role of typhoid toxin in Salmonella Typhi virulence. Yale J. Biol. Med. 2017, 90, 283–290.
- Tamamura, Y.; Tanaka, K.; Uchida, I. Characterization of pertussis-like toxin from Salmonella spp. that catalyzes ADP-ribosylation of G proteins. Sci. Rep. 2017, 7, 2653.
- Miller, R.A.; Betteken, M.I.; Guo, X.; Altier, C.; Duhamel, G.E.; Wiedmann, M. The typhoid toxin produced by the Non-typhoidal Salmonella enterica Serotype javiana is required for induction of a DNA damage response in Vitro and systemic spread in Vivo. MBio 2018, 9, e00467-18.
- Cheng, R.A.; Wiedmann, M. The ADP-ribosylating toxins of Salmonella. Toxins 2019, 11, 416.
- Outbreaks of Salmonella Infections Linked to Backyard Poultry. Final Update; 2019. Available online: https://www.cdc.gov/salmonella/backyardpoultry-05-19/index.html/ (accessed on 2 March 2020).
- CDC 2011. National Enteric Disease Surveillance: Salmonella Annual Summary; US Department of Health and Human Services, CDC: Atlanta, GA, USA, 2011.
- Gebreyes, W.A.; Thakur, S. Multidrug-resistant Salmonella enterica serovar Muenchen from pigs and humans and potential interserovar transfer of antimicrobial resistance. Antimicrob. Agents. Chemother. 2005, 49, 503–511.
- Srinivasan, A. Antibiotic stewardship: Why we must, how we can. Clevel. Clin. J. Med. 2017, 84, 673–679.
- Selvin, J.; Maity, D.; Sajayan, A.; Kiran, G.S. Revealing antibiotic resistance in therapeutic and dietary probiotic supplements. J. Glob. Antimicrob. Resist. 2020, 22, 202–205.
- Xiang, Y.; Li, F.; Dong, N.; Tian, S.; Zhang, H.; Du, X.; Zhou, X.; Xu, X.; Yang, H.; Xie, J.; et al. Investigation of a Salmonellosis Outbreak Caused by Multidrug Resistant Salmonella Typhimurium in China. Front. Microbiol. 2020, 11, 1–12.
- Wang, Z.; Xu, H.; Tang, Y.; Li, Q.; Jiao, X. A Multidrug-resistant Monophasic Salmonella bla CTX-M-14 in a Transferable IncHI2 Plasmid from a Healthy Catering Worker in China. Infect. Drug. Resist. 2020, 13, 3569–3574.
- Besser, J.M. Salmonella epidemiology: A whirlwind of change. Food Microbiol. 2018, 71, 55–59.
- Aydindogan, E.; Guler Celik, E.; Timur, S. Paper-Based Analytical Methods for Smartphone Sensing with Functional Nanoparticles: Bridges from Smart Surfaces to Global Health. Anal. Chem. 2018, 90, 12325–12333.
- Kanchi, S.; Sabela, M.I.; Mdluli, P.S.; Inamuddin; Bisetty, K. Smartphone based bioanalytical and diagnosis applications: A review. Biosens. Bioelectron. 2018, 102, 136–149.
- Yang, Q.; Domesle, K.J.; Ge, B. Loop-Mediated Isothermal Amplification for Salmonella Detection in Food and Feed: Current Applications and Future Directions. Foodborne Pathog. Dis. 2018, 15, 309–331.
- McLain, J.E.; Cytryn, E.; Durso, L.M.; Young, S. Culture-based Methods for Detection of Antibiotic Resistance in Agroecosystems: Advantages, Challenges, and Gaps in Knowledge. J. Environ. Qual. 2016, 45, 432–440.
- Nair, D.V.T.; Thomas, J.V.; Dewi, G.; Johnson, T.; Noll, S.; Cardona, C.; Kollanoor-Johny, A. Effects of Multiple Alternatives-To-Antibiotic Interventions on Multidrug-Resistant Salmonella Heidelberg in Turkey Poults. In Proceedings of the 2017 PSA Annual Meeting, Orlando, FL, USA, 17–20 July 2017; Volume 96, p. 24.
- Carrique-Mas, J.J.; Davies, R.H. Sampling and bacteriological detection of Salmonella in poultry and poultry premises: A review. Rev. Sci. Tech. 2008, 27, 665–677.
- Shah, D.H.; Casavant, C.; Hawley, Q.; Addwebi, T.; Call, D.R.; Guard, J. Salmonella Enteritidis strains from poultry exhibit differential responses to acid stress, oxidative stress, and survival in the egg albumin. Foodborne Pathog. Dis. 9, 258–264.
- Brown, E.; Dessai, U.; Mcgarry, S.; Gerner-Smidt, P. Use of Whole-Genome Sequencing for Food Safety and Public Health in the United States. Foodborne Pathog. Dis. 2019, 16, 441–450.
- Gupta, S.K.; Sharma, P.; McMillan, E.A.; Jackson, C.R.; Hiott, L.M.; Woodley, T.; Humayoun, S.B.; Barrett, J.B.; Frye, J.G.; McClelland, M. Genomic comparison of diverse Salmonella serovars isolated from swine. PLoS ONE 2019, 14, e0224518.
- Almeida, F.; Seribelli, A.A.; Cazentini Medeiros, M.I.; Rodrigues, D.d.P.; de MelloVarani, A.; Luo, Y.; Allard, M.W.; Falcão, J.P. Phylogenetic and antimicrobial resistance gene analysis of Salmonella Typhimurium strains isolated in Brazil by whole genome sequencing. PLoS ONE 2018, 13, e0201882.
- Garcia-Migura, L.; Sunde, M.; Karlsmose, S.; Veldman, K.; Schroeter, A.; Guerra, B.; Granier, S.A.; Perrin-Guyomard, A.; Gicquel-Bruneau, M.; Franco, A.; et al. Establishing streptomycin epidemiological cut-off values for Salmonella and Escherichia coli. Microb. Drug Resist. 2012, 18.
- McDermott, P.F.; Tyson, G.H.; Kabera, C.; Chen, Y.; Li, C.; Folster, J.P.; Ayers, S.L.; Lam, C.; Tate, H.P.; Zhao, S. Whole-genome sequencing for detecting antimicrobial resistance in non-typhoidal Salmonella. Antimicrob. Agents Chemother. 2016, 60, 5515–5520.
- Carroll, L.M.; Wiedmann, M.; den Bakker, H.; Siler, J.; Warchocki, S.; Kent, D.; Lyalina, S.; Davis, M.; Sischo, W.; Besser, T.; et al. Whole-Genome Sequencing of Drug-Resistant Salmonella enterica Isolates from Dairy Cattle and Humans in New York and Washington States Reveals Source and Geographic Associations. Appl. Environ. Microbiol. 2017, 83, e00140-17.
- Mensah, N.; Tang, Y.; Cawthraw, S.; Abuoun, M.; Fenner, J.; Thomson, N.R.; Mather, A.E.; Petrovska-Holmes, L. Determining antimicrobial susceptibility in Salmonella enterica serovar Typhimurium through whole genome sequencing: A comparison against multiple phenotypic susceptibility testing methods. BMC Microbiol. 2019, 19, 148.
- Zwe, Y.H.; Chin, S.F.; Kohli, G.S.; Aung, K.T.; Yang, L.; Yuk, H.G. Whole genome sequencing(WGS) fails to detect antimicrobial resistance (AMR) from heteroresistant subpopulation of Salmonella enterica. Food Microbiol. 2020, 91, 103530.
- Hawkey, J.; le Hello, S.; Doublet, B.; Granier, S.A.; Hendriksen, R.S.; Florian Fricke, W.; Ceyssens, P.J.; Gomart, C.; Billman-Jacobe, H.; Holt, K.E.; et al. Global phylogenomics of multidrug-resistant salmonella enterica serotype kentucky ST198. Microb. Genom. 2019, 5, e000269.
- European Committee on Antimicrobial Susceptibility Testing. MIC and Zone Diameter Distributions and ECOFFs. EUCAST, 2018. Available online: http://www.eucast.org/mic_distributions_and_ecoffs/ (accessed on 24 December 2020).
- Choi, J.R.; Yong, K.W.; Choi, J.Y.; Cowie, A.C. Emerging point-of-care technologies for food safety analysis. Sensors 2019, 19, 817.
- Kim, S.; Masum, F.; Jeon, J.S. Recent Developments of Chip-based Phenotypic Antibiotic Susceptibility Testing. Biochip J. 2019.
- Hu, Q.; Yu, Y.; Gu, D.; Xie, L.; Chen, X.; Xu, N.; Ruan, J.J.; Dowson, C.; Ruan, B.H. Detection of “hidden” Antimicrobial Drug Resistance. ACS Infect. Dis. 2019, 5, 1252–1263.
- Garner, O.; Traczewski, M.M.; Beasley, D.; Harrington, A.; DesJarlais, S.; Hastey, C.; Brookman, R.; Lockett, Z.; Chau, J. 667. Multicenter Assessment of Enterobacterales, Salmonella spp. and Pseudomonas aeruginosa Using Updated CLSI Levofloxacin Breakpoints on MicroScan Dried Gram-Negative MIC Panels. Open Forum Infect. Dis. 2020, 7 (Suppl. S1), S388–S389.
- Leonard, H.; Colodner, R.; Halachmi, S.; Segal, E. Recent Advances in the Race to Design a Rapid Diagnostic Test for Antimicrobial Resistance. ACS Sens. 2018, 3, 2202–2217.
- Adan, A.; Alizada, G.; Kiraz, Y.; Baran, Y.; Nalbant, A. Flow cytometry: Basic principles and applications. Crit. Rev. Biotechnol. 2017, 37, 163–176.
- Ambriz-Aviña, V.; Contreras-Garduño, J.A.; Pedraza-Reyes, M. Applications of Flow Cytometry to Characterize Bacterial Physiological Responses. BioMed Res. Int. 2014, 461941.
- Van Meervenne, E.; van Coillie, E.; Kerckhof, F.M.; Devlieghere, F.; Herman, L.; de Gelder, L.S.P.; Top, E.M.; Boon, N. Strain-specific transfer of antibiotic resistance from an environmental plasmid to foodborne pathogens. J. Biomed. Biotechnol. 2012, 834598.
- Huang, T.H.; Tzeng, Y.L.; Dickson, R.M. FAST: Rapid determinations of antibiotic susceptibility phenotypes using label-free cytometry. Cytom. Part A. 2018, 93, 639–648.
- Rishi, P.; Bhagat, N.R.; Thakur, R.; Pathania, P. Tackling Salmonella Persister Cells by Antibiotic-Nisin Combination via Mannitol. Indian J. Microbiol. 2018, 58, 239–243.
- Claudi, B.; Spröte, P.; Chirkova, A.; Personnic, N.; Zankl, J.; Schürmann, N.; Schmidt, A.; Bumann, D. Phenotypic variation of Salmonella in host tissues delays eradication by antimicrobial chemotherapy. Cell 2014, 158, 722–733.
- Vohra, P.; Vrettou, C.; Hope, J.C.; Hopkins, J.; Stevens, M.P. Nature and consequences of Interactions between Salmonella enterica serovar Dublin and host cells in cattle. Vet. Res. 2019, 50, 99.
- Tsolis, R.M.; Adams, L.G.; Ficht, T.A.; Baumler, A.J. Contribution of Salmonella typhimurium virulence factors to diarrheal disease in calves. Infect. Immun. 1999, 67, 4879–4885.
- Andrade, F.F.; Gomes, R.; Martins-Oliveira, I.; Dias, A.; Rodrigues, A.G.; Pina-Vaz, C. A Rapid Flow Cytometric Antimicrobial Susceptibility Assay (FASTvet) for Veterinary Use-Preliminary Data. Front. Microbiol. 2020, 11, 1944.
- Jamerlan, A.; An, S.S.A.; Hulme, J. Advances in amyloid beta oligomer detection applications in Alzheimer’s disease. TrAC Trends Anal. Chem. 2020, 129, 115919.
- Giau, V.V.; An, S.S.A.; Hulme, J. Recent advances in the treatment of pathogenic infections using antibiotics and nano-drug delivery vehicles. Drug Des. Devel. Ther. 2019, 13, 327–343.
- Paniel, N.; Noguer, T. Detection of Salmonella in food matrices, from conventional methods to recent aptamer-sensing technologies. Foods 2019, 8, 371.
- Khan, S.A.; Singh, A.K.; Senapati, D.; Fan, Z.; Ray, P.C. Bio-conjugated popcorn shaped gold nanoparticles for targeted photothermal killing of multiple drug resistant Salmonella DT104. J. Mater. Chem. 2011, 21, 17705–17709.
- Jia, M.; Liu, Z.; Wu, C.; Zhang, Z.; Ma, L.; Lu, X.; Mao, Y.; Zhang, H. Detection of Escherichia coli O157:H7 and Salmonella enterica serotype Typhimurium based on cell elongation induced by beta-lactam antibiotics. Analyst 2019, 144, 4505–4512.
- Lin, Y.; Hamme, A.T. Targeted highly sensitive detection/eradication of multidrug resistant Salmonella DT104 through gold nanoparticle-SWCNT bioconjugated nanohybrids. Analyst 2014, 139, 3702–3705.
- Yang, K.; Li, H.Z.; Zhu, X.; Su, J.Q.; Ren, B.; Zhu, Y.G.; Cui, L. Rapid Antibiotic Susceptibility Testing of Pathogenic Bacteria Using Heavy-Water-Labeled Single-Cell Raman Spectroscopy in Clinical Samples. Anal. Chem. 2019, 91, 6296–6303.
- Zeinhom, M.M.A.; Wang, Y.; Sheng, L.; Du, D.; Li, L.; Zhu, M.J.; Lin, Y. Smart phone based immunosensor coupled with nanoflower signal amplification for rapid detection of Salmonella Enteritidis in milk, cheese and water. Sens. Actuators B Chem. 2018, 261, 75–82.
- Pramanik, A.; Davis, D.; Patibandla, S.; Begum, S.; Ray, P.; Gates, K.; Gao, Y.; Chandra Ray, P. A WS2-gold nanoparticle heterostructure-based novel SERS platform for the rapid identification of antibiotic-resistant pathogens. Nanoscale Adv. 2020, 2025–2033.
