Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Antibiotic resistance is a significant global health concern that affects both human and animal populations. The One Health approach acknowledges the interconnectedness of human health, animal health, and the environment. It emphasizes the importance of collaboration and coordination across these sectors to tackle complex health challenges such as antibiotic resistance.
- antimicrobial resistance
- antibiotic resistance
- One Health
1. Examining the Global Impact of Antibiotic Resistance
Antimicrobial resistance (AMR) in pathogenic bacteria can be defined in two ways: microbiologically and clinically. Microbiological resistance is determined by the presence of a genetically acquired or mutated resistance mechanism in the pathogen, which is classified as resistant, intermediate, or susceptible based on specific cut-off values in laboratory tests [1]. Clinical resistance is defined by the level of antimicrobial activity that is associated with a high likelihood of treatment failure. Specifically, it means that using a drug against which the pathogen was tested and proved susceptible is more effective than using a drug against which the pathogen was tested and proved resistant. The cut-off values for clinical resistance may vary depending on factors such as the site of infection or the dosage of the drug [2].
AMR in bacterial pathogens is a significant global concern associated with high rates of illness and mortality [3][4]. Several factors increase the demands for antibiotic prescriptions, as well as situations that create financial incentives from medication distribution, such as unregulated access and usage of over-the-counter drugs, can lead to inappropriate prescribing of antimicrobials. Both Gram-positive and Gram-negative bacteria have developed multidrug resistant patterns, leading to challenging and sometimes infections that are untreatable with conventional antimicrobials. In many healthcare settings, there is a lack of early identification of causative microorganisms and their susceptibility to antimicrobials in patients with serious infections, leading to the excessive and unnecessary use of broad-spectrum antibiotics [3][4]. Consequently, there has been a notable increase in emerging resistance. Coupled with poor infection control practices, resistant bacteria can easily be disseminated to other patients and the environment [3][4].
The introduction of antibiotics as clinical agents radically changed the evolution and spread of resistance by providing considerable selective pressures, especially on members of the microbiota of humans and domestic animals but also in environments heavily polluted with antibiotics. This selective pressure has promoted the mobilization and horizontal transfer of a large range of antibiotic resistance genes (ARGs) among many bacterial species, particularly to those causing disease. The downstream consequences of such accumulating evolutionary events are gradually increasing the difficulty in the prevention and treatment of bacterial infections. As bacteria and mobile genetic elements often cross environments and species boundaries, it is critical to understand and acknowledge the connections between the human, animal, and environmental microbiota (the One Health Concept [5][6]) (https://www.cdc.gov/onehealth/ accessed on 1 July 2023) to manage this global health challenge [7][8][9][10][11][12][13][14][15][16][17] (Figure 1).
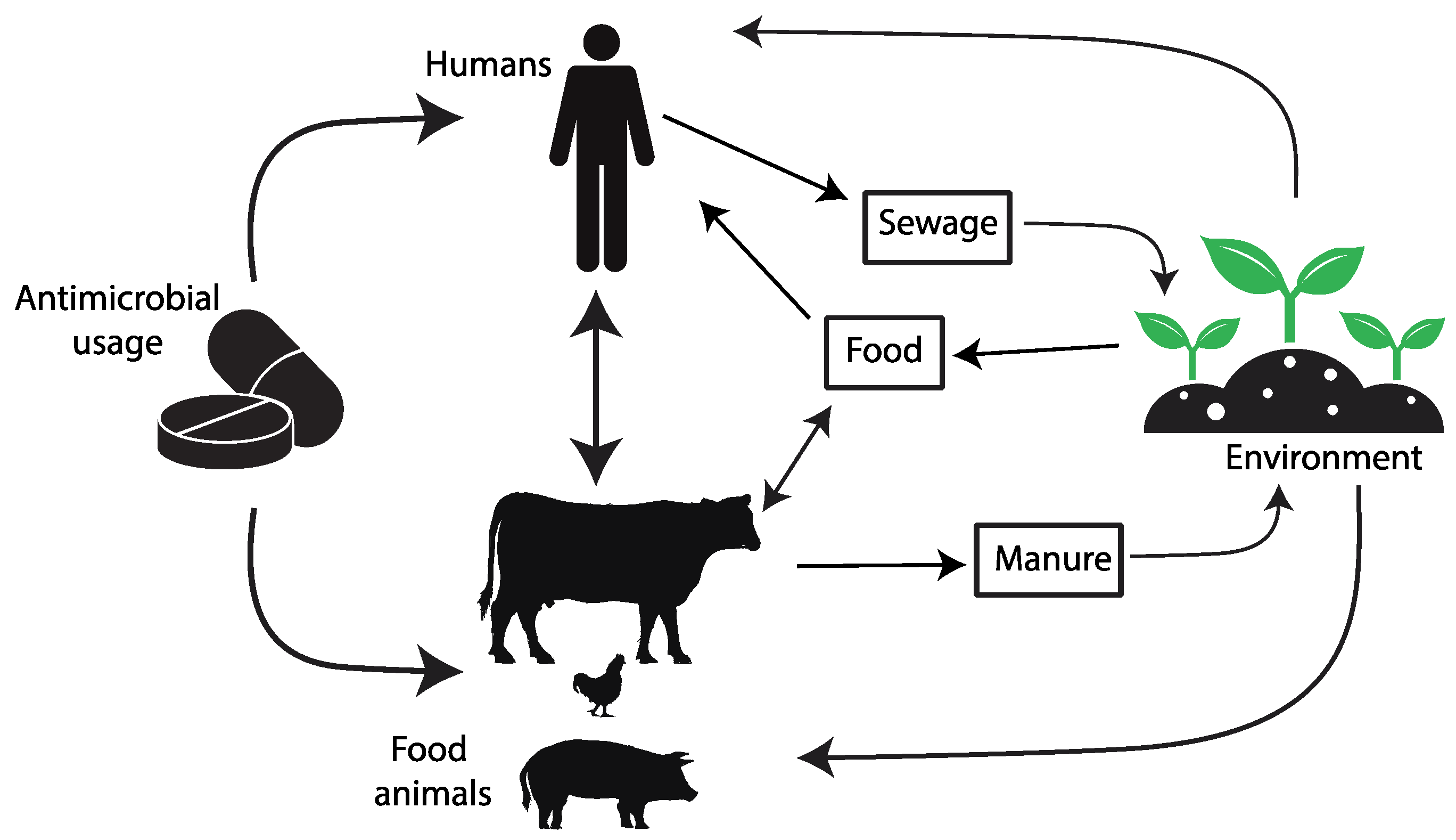
Figure 1. The diagram depicts the pathways of transmission for antimicrobial resistance between farm animals, the surrounding environment, and human populations.
The threats posed by AMR are of increasing concern, even in low- and middle-income countries (LMICs), as the rates of antibiotic use increase. Assessing the extent of AMR in LMICs is complicated due to inadequate surveillance programs, though efforts like the WHO’s GLASS project (2015) have been initiated [18]. The misuse of antibiotics in humans, animals, and crops, along with the mismanagement of pharmaceutical waste during production, drives AMR in LMICs. Factors like poor sanitation, limited healthcare access, and lax regulations contribute to AMR spread. The COVID-19 pandemic compounded these challenges, increasing antibiotic misuse in LMICs and diverting attention from the AMR threat [19]. AMR hotspots are seen in parts of India, China, Pakistan, and other regions. Emerging resistance is noted in Kenya, Morocco, and Uruguay. Survey coverage did not always match hotspots. Common antimicrobial classes in animal production had high resistance rates [20]. Through the active participation of various countries in the WHO’s GLASS project, valuable insights have been gleaned regarding the total consumption of antibiotic, measured in terms of Defined Daily Doses (DDD) per 1000 inhabitants per day [18] (Figure 2). However, it is important to acknowledge that these findings are limited due to the lack of universal public engagement in the specific country data collection process.
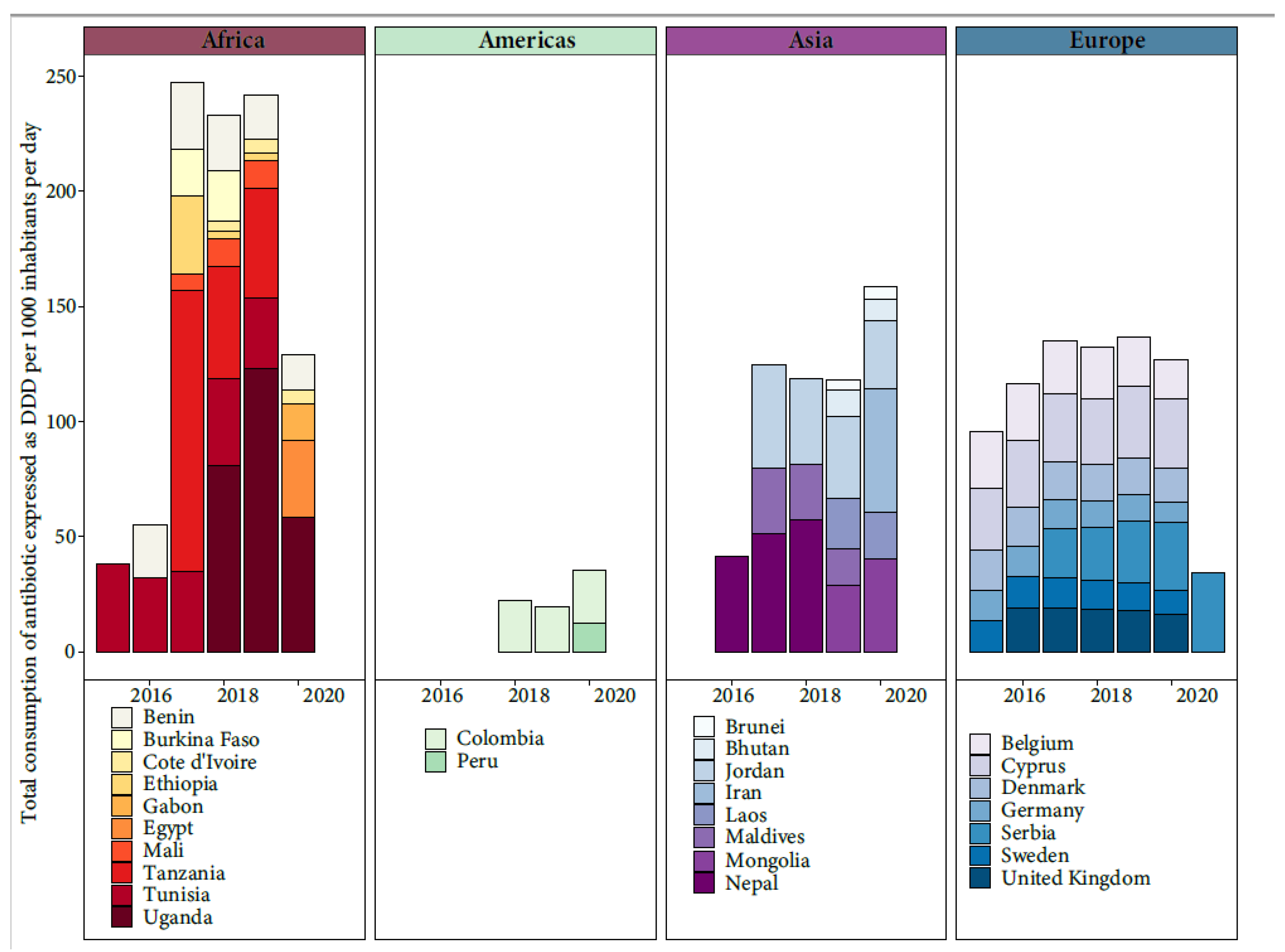
Figure 2. Total consumption of antibiotic expressed as Defined Daily Doses (DDD) per 1000 inhabitants per day from the available data of WHO’s GLASS project.
Treating antibiotic resistant infections usually requires the prolonged use of last-line antibiotics, and these approaches often fail, contributing to infection persistence [21]. Several factors contribute to the development and spread of antibiotic resistance [22], and among these are:
-
Incomplete Treatment: When individuals do not complete their prescribed antibiotic course, it creates an environment for the survival of the resistant bacteria, leading to the proliferation of resistant strains.
-
Use in Agriculture: Antibiotics are extensively used in livestock and agriculture to promote growth and prevent infections in animals. This widespread use contributes to the development of resistant bacteria that can be transmitted to humans through the food chain. In addition, mobile elements conferring antibiotic resistance can move from livestock-associated lineages to human lineages [28][29].
-
Global Travel and Trade: Resistant bacteria can spread quickly across borders through international travel and trade, making antibiotic resistance a global issue [30].
-
Lack of New Antibiotics: The development of new antibiotics has slowed significantly in recent decades, reducing the arsenal of drugs available to combat resistant infections [31].
2. Antibiotic Resistance: A Threat to Human and Animal Health
Over the past few decades, the indiscriminate and inappropriate use of antibiotics has led to the emergence of increasingly resistant bacterial strains, rendering previously effective drugs ineffective in treating infections [3]. This phenomenon poses a significant challenge to modern medicine as it compromises the ability to treat a wide range of infectious diseases, putting the lives of millions of people and animals at risk, and demanding immediate and concerted action to address this critical global challenge. Because of the interconnectedness of human and animal health, a unified approach known as One Health, which recognizes the inextricable link between human, animal, and environmental health, is needed [32] (Figure 1).
Diseases that were once easily treatable with antibiotics, such as pneumonia, urinary tract infections, and skin infections, now become formidable adversaries, necessitating more aggressive and costly treatment approaches. The loss of effective antibiotics not only jeopardizes the treatment of common infections but also undermines critical medical procedures such as surgeries, organ transplants, and cancer treatments, where the availability of effective antimicrobial agents is essential [33][34]. Similarly, animal health is significantly impacted by antibiotic resistance. In veterinary medicine, the diminished efficacy of antibiotics against resistant bacteria poses challenges in treating and controlling infectious diseases in livestock, companion animals, and wildlife [35]. This not only compromises animal welfare but also raises concerns regarding zoonotic diseases, where antibiotic-resistant bacteria can be transmitted from animals to humans. Livestock-associated antibiotic resistance can enter the food chain, leading to the consumption of antibiotic-resistant bacteria through animal-derived food products, further contributing to the spread of resistance and posing risks to human health [36][37][38].
This interplay between human and animal health cannot be overlooked. The data indicate that, among the countries presently utilizing veterinary antibiotics, five nations are predicted to experience the most significant percentage growth by 2030. These countries are Myanmar and Indonesia, followed by Nigeria and Peru, with Vietnam also showing a moderate increase [20]. To effectively combat antibiotic resistance, collaboration is paramount among stakeholders from various sectors, including healthcare professionals, veterinarians, researchers, policymakers, and the public. This is essential to optimize animal care, reduce the selection pressure for antimicrobial resistance, and ensure continued access to vital antimicrobial agents. Resolving this complex issue does not have straightforward solutions, but veterinarians must carefully consider the implications of their daily decisions and strive to optimize antimicrobial use for the benefit of their patients and society. Robust surveillance systems should be established to monitor resistance patterns in both human and animal populations, allowing for early detection and response [39]. Several studies have shown that aquatic bacteria resistant to specific antibiotics commonly found with E. coli, a human pathogen, shared some genetic elements and determinants with human and animal bacteria [40][41]. Responsible antibiotic use must be promoted, emphasizing appropriate prescribing practices in healthcare settings, and implementing guidelines for antibiotic use in agriculture. Eliminating human antibacterial use can potentially result in a more significant reduction (65.7–99.7%) in the colonization of resistant bacteria over 20 years [42]. On a smaller scale, reducing human-to-human transmission has the potential to reduce the AMR by 8.2–36.3% [42]. Strengthening infection control measures in healthcare facilities and animal farming is crucial in minimizing the spread of resistant bacteria [43]. Furthermore, substantial investment in research and development is necessary to drive innovation in the field of antibiotics. Efforts should focus on discovering new antibiotics, exploring alternative treatment strategies, such as phage therapy, nanoparticles, probiotics, and immunotherapies, and developing rapid diagnostic tools to facilitate targeted treatment. Education and awareness campaigns play a pivotal role in fostering a greater understanding of antibiotic resistance among healthcare providers, veterinarians, policymakers, and the public. By promoting behavioral changes that prioritize judicious antibiotic use and infection prevention, researchers can collectively combat the spread of antibiotic resistance. The challenges posed by antibiotic resistance demand a multifaceted and sustained effort. International collaboration is crucial in sharing knowledge, resources, and best practices to address this global crisis. Additionally, policy interventions are necessary to regulate the use of antibiotics in both human and animal health sectors, ensuring their prudent and responsible use. Investments in research and development should be incentivized to encourage the discovery of new antimicrobial agents and the development of innovative therapeutic approaches [44].
3. Combating Antimicrobial Resistance: A One Health Approach for Global Health Security
Due to the increasing development of multidrug-resistant bacteria, which renders existing antimicrobials ineffective in treating illnesses caused by these bacteria, AMR is a major global concern [4]. This predicament emphasizes the critical need for innovative treatments. Ineffective antimicrobials have serious effects, including increased mortality rates, as antimicrobial-resistant microbes grow increasingly common [34]. Addressing AMR necessitates a holistic approach known as One Health, which entails creating, implementing, and monitoring AMR surveillance programs, policies, and research. To achieve improved public health results, the One Health strategy encourages intersectoral collaboration among the public health, animal health, and environmental health sectors. Antibiotic stewardship is important in the One Health paradigm because it emphasizes ethical antibiotic use among healthcare providers, veterinarians, and farmers [45]. Adhering to antibiotic-use guidelines and policies helps to lessen the selective pressure that develops antibiotic resistance. Another important feature of the One Health strategy is interdisciplinary research, which allows for a thorough knowledge of the complex dynamics of antibiotic resistance [32]. By incorporating findings from a variety of domains, including human and veterinary medicine, ecology, microbiology, and others, it will be possible to obtain a thorough picture of how antibiotic resistance develops, spreads, and can be tackled by merging knowledge from several areas. This comprehension drives the development of novel tactics such as new diagnostic tools, alternative therapies, and vaccinations. The One Health approach also argues for comprehensive policies to be implemented at the national and international levels [32]. While some antimicrobial classes, such as those used to treat tuberculosis or illnesses uncommon in animals, are primarily designated for human usage, most antimicrobial classes are utilized in both people and animals. This includes the use of these chemicals in domestic mammals, birds, farmed fish, and shellfish, honeybees, and other creatures [46][47][48][49][50]. Certain antimicrobials are used in horticulture on occasion to treat and prevent bacterial diseases in fruits, such as “fire blight” in apples and pears caused by Erwinia amylovora [32]. Antimicrobials are used in human medicine to treat clinical infections in patients, with limited prophylactic usage for individuals or groups (e.g., post-surgery or post-exposure prophylaxis for vaccine preventable diseases). Antimicrobial use differs significantly between companion animals (e.g., dogs, cats, pet birds, horses) and food-producing animals, according to veterinary medicine [51]. Companion animals receive antimicrobial treatments in a manner comparable to humans. Antibiotics are predominantly prescribed for individual animals to address clinical illnesses, with occasional preventive use, such as after surgeries [52]. When some animals in a group require treatment for clinical infection, antimicrobial therapy is frequently delivered to the entire group through feed or water, even if most animals show no indications of infection. This method, recognized in the animal health industry as “therapeutic” use, varies from therapeutic use in human medicine. Furthermore, antimicrobials are used in food animals to treat clinically unwell animals, such as dairy cows with mastitis. The term “metaphylaxis” refers to group-level therapeutic and/or preventative treatment that includes the mass delivery of therapeutic doses of an antibiotic to a high-risk group of animals [53]. Because of the increased risk of bovine respiratory disease, one example of metaphylaxis is the administration of injectable antimicrobials to groups of calves upon their arrival at a feedlot. Thus, adopting a One Health approach is crucial in AMR for the sake of global health security. The rapid spread of multidrug-resistant bacteria and the ineffectiveness of existing antimicrobials highlight the urgent need for innovative treatments. The One Health approach places antibiotic stewardship at its core, recognizing its vital importance and direct impact on reducing antibiotic resistance [54][55]. Antibiotic stewardship programs constitute a fundamental component of preventive strategies. These programs aim to optimize antibiotic use by promoting judicious practices to mitigate the development of resistance by: (i) ensuring that antibiotics are employed only when absolutely necessary, (ii) selecting the most appropriate antibiotic for each specific situation, and (iii) administering it in the proper dosage for the appropriate duration [56]. This approach centers on responsible antibiotic use among various stakeholders, such as governments, healthcare providers, veterinarians, researchers, pharmaceutical companies, and farmers. By adhering to guidelines and policies pertaining to responsible antibiotic usage, researchers can reduce the selective pressure that drives antibiotic resistance. This stewardship should be embraced across all the involved stakeholders and be accompanied by educational campaigns and robust policy frameworks to support its successful implementation. In more detail, global initiatives, such as the World Health Organization’s Global Antimicrobial Resistance Surveillance System (GLASS) (https://www.who.int/initiatives/glass, accessed on 15 July 2023), demonstrate the global effort to combat antibiotic resistance and the importance of international cooperation. The national action plan for combating antibiotic-resistant bacteria, developed by US Department of Health and Human services (HHS), is a 2020–2025 plan [57] including the One Health approach as effective plan. The first objective is to identify and implement measures to foster stewardship of antibiotics in animals, eliminate the use of medically important antibiotics for growth promotion in animals, and bring under veterinary oversight other uses of medically important antibiotics. By year 5, new regulations were already implemented and antibiotic drugs used for feed transitioned from over-the-counter to Veterinary Feed Directive (VFD) or prescription status [58]. Likewise, the USDA and FDA collaborated to create, execute, and assess educational outreach efforts aimed at promoting responsible antibiotic use and stewardship. This involved interacting with livestock and veterinary associations to engage relevant stakeholders and ensure effective communication [58].
This entry is adapted from the peer-reviewed paper 10.3390/pathogens12091074
References
- CLSI. Appropriate Reference Is—Performance Standards for Antimicrobial Susceptibility Testing; Approved Standard M100-S20; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2020.
- MacGowan, A.; Macnaughton, E. Antibiotic resistance. Medicine 2017, 45, 622–628.
- Akova, M. Epidemiology of antimicrobial resistance in bloodstream infections. Virulence 2016, 7, 252–266.
- Frieri, M.; Kumar, K.; Boutin, A. Antibiotic resistance. J. Infect. Public Health 2017, 10, 369–378.
- Buschhardt, T.; Günther, T.; Skjerdal, T.; Torpdahl, M.; Gethmann, J.; Filippitzi, M.-E.; Maassen, C.; Jore, S.; Ellis-Iversen, J.; Filter, M. A one health glossary to support communication and information exchange between the human health, animal health and food safety sectors. One Health 2021, 13, 100263.
- Mackenzie, J.S.; Jeggo, M. The One Health Approach—Why Is It So Important? Trop. Med. Infect. Dis. 2019, 4, 88.
- Larsson, D.G.J.; Flach, C.-F. Antibiotic resistance in the environment. Nat. Rev. Genet. 2021, 20, 257–269.
- Berendonk, T.U.; Manaia, C.M.; Merlin, C.; Fatta-Kassinos, D.; Cytryn, E.; Walsh, F.; Bürgmann, H.; Sørum, H.; Norström, M.; Pons, M.N.; et al. Tackling Antibiotic Resistance: The Environmental Framework. Nat. Rev. Microbiol. 2015, 13, 310–317.
- Wellington, E.M.H.; Boxall, A.B.A.; Cross, P.; Feil, E.J.; Gaze, W.H.; Hawkey, P.M.; Johnson-Rollings, A.S.; Jones, D.L.; Lee, N.M.; Otten, W.; et al. The role of the natural environment in the emergence of antibiotic resistance in gram-negative bacteria. Lancet Infect. Dis. 2013, 13, 155–165.
- Bengtsson-Palme, J.; Larsson, D.G.J. Antibiotic resistance genes in the environment: Prioritizing risks. Nat. Rev. Microbiol. 2015, 13, 396.
- Chow, L.K.M.; Ghaly, T.M.; Gillings, M.R. A survey of sub-inhibitory concentrations of antibiotics in the environment. J. Environ. Sci. 2021, 99, 21–27.
- Andersson, D.I.; Balaban, N.Q.; Baquero, F.; Courvalin, P.; Glaser, P.; Gophna, U.; Kishony, R.; Molin, S.; Tønjum, T. Antibiotic resistance: Turning evolutionary principles into clinical reality. FEMS Microbiol. Rev. 2020, 44, 171–188.
- Singer, A.C.; Shaw, H.; Rhodes, V.; Hart, A. Review of antimicrobial resistance in the environment and its relevance to environmental regulators. Front. Microbiol. 2016, 7, 1728.
- Graham, D.W.; Bergeron, G.; Bourassa, M.W.; Dickson, J.; Gomes, F.; Howe, A.; Kahn, L.H.; Morley, P.S.; Scott, H.M.; Sim-jee, S.; et al. Complexities in understanding antimicrobial resistance across domesticated animal, human, and environmental systems. Ann. N. Y. Acad. Sci. 2019, 1441, 17–30.
- Ivančić, I.; Paliaga, P.; Pfannkuchen, M.; Djakovac, T.; Najdek, M.; Steiner, P.; Korlević, M.; Markovski, M.; Baričević, A.; Tanković, M.S.; et al. Environmental dimensions of antibiotic resistance: Assessment of basic science gaps. FEMS Microbiol. Ecol. 2018, 94, fiy195.
- United Nations Environment Programme. Frontiers 2017: Emerging Issues of Environmental Concern. 2017. Available online: https://www.unep.org/resources/frontiers-2017-emerging-issues-environmental-concern (accessed on 23 July 2023).
- European Parliament. Strategic Approach to Pharmaceuticals in the Environment; European Parliament: Strasbourg, France, 2020.
- Global Antimicrobial Resistance and Use Surveillance System (GLASS). Available online: https://www.who.int/initiatives/glass (accessed on 14 August 2023).
- Sulis, G.; Sayood, S.; Gandra, S. Antimicrobial resistance in low- and middle-income countries: Current status and future directions. Expert Rev. Anti-Infect. Ther. 2021, 20, 147–160.
- Van Boeckel, T.P.; Pires, J.; Silvester, R.; Zhao, C.; Song, J.; Criscuolo, N.G.; Gilbert, M.; Bonhoeffer, S.; Laxminarayan, R. Global trends in antimicrobial resistance in animals in low- and middle-income countries. Science 2019, 365, eaaw1944.
- Ribeiro, S.M.; Felício, M.R.; Boas, E.V.; Gonçalves, S.; Costa, F.F.; Samy, R.P.; Santos, N.C.; Franco, O.L. New frontiers for anti-biofilm drug development. Pharmacol. Ther. 2016, 160, 133–144.
- Lipsitch, M.; Samore, M.H. Antimicrobial use and antimicrobial resistance: A population perspective. Emerg. Infect. Dis. 2002, 8, 347–354.
- Karakonstantis, S.; Kalemaki, D. Antimicrobial overuse and misuse in the community in Greece and link to antimicrobial resistance using methicillin-resistant S. aureus as an example. J. Infect. Public Health 2019, 12, 460–464.
- English, B.K.; Gaur, A.H. The use and abuse of antibiotics and the development of antibiotic resistance. Adv. Exp. Med. Biol. 2010, 659, 73–82.
- Chang, Y.; Chusri, S.; Sangthong, R.; McNeil, E.; Hu, J.; Du, W.; Li, D.; Fan, X.; Zhou, H.; Chongsuvivatwong, V.; et al. Clinical pattern of antibiotic overuse and misuse in primary healthcare hospitals in the southwest of China. PLoS ONE 2019, 14, e0214779.
- Ray, M.J.; Tallman, G.B.; Bearden, D.T.; Elman, M.R.; McGregor, J.C. Antibiotic prescribing without documented indication in ambulatory care clinics: National cross sectional study. BMJ 2019, 367, l6461.
- Fleming-Dutra, K.E.; Hersh, A.L.; Shapiro, D.J.; Bartoces, M.; Enns, E.A.; File, T.M.; Finkelstein, J.A.; Gerber, J.S.; Hyun, D.Y.; Linder, J.A.; et al. Prevalence of Inappropriate Antibiotic Prescriptions Among US Ambulatory Care Visits, 2010–2011. JAMA 2016, 315, 1864–1873.
- Martin, M.J.; Thottathil, S.E.; Newman, T.B. Antibiotics Overuse in Animal Agriculture: A Call to Action for Health Care Providers. Am. J. Public Health 2015, 105, 2409–2410.
- Chang, Q.; Wang, W.; Regev-Yochay, G.; Lipsitch, M.; Hanage, W.P. Antibiotics in agriculture and the risk to human health: How worried should we be? Evol. Appl. 2015, 8, 240–247.
- Acar, J.; Röstel, B. Antimicrobial resistance: An overview. Rev. Sci. Tech. 2001, 20, 797–810.
- WHO. Lack of Innovation Set to Undermine Antibiotic Performance and Health Gains. 2022. Available online: https://www.who.int/news/item/22-06-2022-22-06-2022-lack-of-innovation-set-to-undermine-antibiotic-performance-and-health-gains (accessed on 8 July 2023).
- McEwen, S.A.; Collignon, P.J. Antimicrobial Resistance: A One Health Perspective. Microbiol. Spectr. 2018, 6, 2.
- Jasovský, D.; Littmann, J.; Zorzet, A.; Cars, O. Antimicrobial resistance-a threat to the world’s sustainable development. Ups. J. Med. Sci. 2016, 121, 159–164.
- Laxminarayan, R.; Duse, A.; Wattal, C.; Zaidi, A.K.M.; Wertheim, H.F.L.; Sumpradit, N.; Vlieghe, E.; Hara, G.L.; Gould, I.M.; Goossens, H.; et al. Antibiotic resistance-the need for global solutions. Lancet Infect. Dis. 2013, 13, 1057–1098.
- Weese, J.S.; Giguère, S.; Guardabassi, L.; Morley, P.S.; Papich, M.; Ricciuto, D.R.; Sykes, J.E. ACVIM consensus statement on therapeutic antimicrobial use in animals and antimicrobial resistance. J. Vet. Intern. Med. 2015, 29, 487–498.
- Vidovic, N.; Vidovic, S. Antimicrobial Resistance and Food Animals: Influence of Livestock Environment on the Emergence and Dissemination of Antimicrobial Resistance. Antibiotics 2020, 9, 52.
- Larsen, J.; Raisen, C.L.; Ba, X.; Sadgrove, N.J.; Padilla-González, G.F.; Simmonds, M.S.J.; Loncaric, I.; Kerschner, H.; Ap-falter, P.; Hartl, R.; et al. Emergence of methicillin resistance predates the clinical use of antibiotics. Nature 2022, 602, 135–141.
- Harrison, E.M.; Coll, F.; Toleman, M.S.; Blane, B.; Brown, N.M.; Török, M.E.; Parkhill, J.; Peacock, S.J. Genomic surveillance reveals low prevalence of livestock-associated methicillin-resistant Staphylococcus aureus in the East of England. Sci. Rep. 2017, 7, 7406.
- Velazquez-Meza, M.E.; Galarde-López, M.; Carrillo-Quiróz, B.; Alpuche-Aranda, C.M. Antimicrobial resistance: One Health approach. Vet. World 2022, 15, 743–749.
- Cabello, F.C.; Godfrey, H.P.; Tomova, A.; Ivanova, L.; Dölz, H.; Millanao, A.; Buschmann, A.H. Antimicrobial use in aquaculture re-examined: Its relevance to antimicrobial resistance and to animal and human. Health Environ. Microbiol. 2013, 15, 1917–1942.
- Welch, T.J.; Evenhuis, J.; White, D.G.; McDermott, P.F.; Harbottle, H.; Miller, R.A.; Griffin, M.; Wise, D. IncA/C plasmid-mediated florfenicol resistance in the catfish pathogen Edwardsiella ictaluri. Antimicrob. Agents Chemother. 2009, 53, 845–846.
- Booton, R.D.; Meeyai, A.; Alhusein, N.; Buller, H.; Feil, E.; Lambert, H.; Mongkolsuk, S.; Pitchforth, E.; Reyher, K.K.; Sakcamduang, W.; et al. One Health drivers of antibacterial resistance: Quantifying the relative impacts of human, animal and environmental use and transmission. One Health 2021, 12, 100220.
- Manyi-Loh, C.; Mamphweli, S.; Meyer, E.; Okoh, A. Antibiotic Use in Agriculture and Its Consequential Resistance in Environmental Sources: Potential Public Health Implications. Mol. A J. Synth. Chem. Nat. Product. Chem. 2018, 23, 795.
- Majumder, M.A.A.; Rahman, S.; Cohall, D.; Bharatha, A.; Singh, K.; Haque, M.; Gittens-St Hilaire, M. Antimicrobial Stewardship: Fighting Antimicrobial Resistance and Protecting Global Public Health. Infect. Drug Resist. 2020, 13, 4713–4738.
- Aslam, B.; Khurshid, M.; Arshad, M.I.; Muzammil, S.; Rasool, M.; Yasmeen, N.; Shah, T.; Chaudhry, T.H.; Rasool, M.H.; Shahid, A.; et al. Antibiotic Resistance: One Health One World Outlook. Front. Cell Infect. Microbiol. 2021, 11, 771510.
- European Medicines Agency. European Survelliance of Veterinary Antimicrobial Consumption. Sales of Veterinary Antimicro-bial Agents in 31 European Countries in 2018. 21 October 2020. Tenth ESVAC report. 21 October.
- ECDC/EFSA/EMA. ECDC/EFSA/EMA first joint report on the integrated analysis of the consumption of antimicrobial agents and occurrence of antimicrobial resistance in bacteria from humans and food-producing animals. EFSA J. 2015, 13.
- Van Boeckel, T.P.; Brower, C.; Gilbert, M.; Grenfell, B.T.; Levin, S.A.; Robinson, T.P. Global trends in antimicrobial use in food animals. Proc. Natl. Acad. Sci. USA 2015, 112, 5649–5654.
- McEwen, S.A.; Fedorka-Cray, P.J. Antimicrobial Use and Resistance in Animals. Clin. Infect. Dis. 2002, 34, S93–S106.
- Food and Agriculture Organization (FAO). Drivers, Dynamics and Epidemiology of Antimicrobial Resistance in Animal Production. In Fao. 2016. Available online: http://www.fao.org/documents/card/es/c/d5f6d40d-ef08-4fcc-866b-5e5a92a12dbf/ (accessed on 30 July 2023).
- Reddy, S.B.; Kumari, N.K. Methicillin-resistant Staphylococcus Aureus (MRSA) isolated from dogs with recurrent pyoderma. J. Dairy Vet. Anim. Res. 2016, 3, 62–65.
- Collignon, P.J.; McEwen, S.A. One Health-Its Importance in Helping to Better Control Antimicrobial Resistance. Trop. Med. Infect. Dis. 2019, 4, 22.
- Abushaheen, M.A.; Muzaheed; Fatani, A.J.; Alosaimi, M.; Mansy, W.; George, M.; Acharya, S.; Rathod, S.; Divakar, D.D.; Jhugroo, C.; et al. Antimicrobial resistance, mechanisms and its clinical significance. Dis. Mon. 2020, 66, 100971.
- Scott, H.M.; Acuff, G.; Bergeron, G.; Bourassa, M.W.; Gill, J.; Graham, D.W.; Kahn, L.H.; Morley, P.S.; Salois, M.J.; Simjee, S.; et al. Critically important antibiotics: Criteria and approaches for measuring and reducing their use in food animal agriculture. Ann. N. Y. Acad. Sci. 2019, 1441, 8–16.
- Landers, T.F.; Cohen, B.; Wittum, T.E.; Larson, E.L. A Review of Antibiotic Use in Food Animals: Perspective, Policy, and Potential. Public Health Rep. 2012, 127, 4–22.
- Zay Ya, K.; Win, P.T.N.; Bielicki, J.; Lambiris, M.; Fink, G. Association Between Antimicrobial Stewardship Programs and Antibiotic Use Globally: A Systematic Review and Meta-Analysis. JAMA Netw. Open. 2023, 6, e2253806.
- U.S. National Action Plan for Combating Antibiotic-Resistant Bacteria|CDC. Available online: https://www.cdc.gov/drugresistance/us-activities/national-action-plan.html (accessed on 15 August 2023).
- National Action Plan for Combating Antibiotic-Resistant Bacteria Progress Report: Year 5|ASPE. Available online: https://aspe.hhs.gov/reports/carb-year-5-report (accessed on 15 August 2023).
This entry is offline, you can click here to edit this entry!
