You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Plant defense depends on constitutive and induced factors combined as defense mechanisms. These mechanisms involve a complex signaling network linking structural and biochemical defense. Antimicrobial and pathogenesis-related (PR) proteins are examples of this mechanism, which can accumulate extra- and intracellular space after infection.
- plant–pathogen interaction
- plant protection
- preformed mechanism
1. PR-2 and PR-3 Families: β-1,3 Glucanases and Chitinases
β-1,3 glucanases belong to PR-2 family, classified as endonuclease enzymes (E.C.3.2.1.39). They are multifunctional enzymes present in many living beings, including bacteria, fungi and some invertebrate animals and plants. Over the years, four β-glucanases subfamilies have been reported (A, B, C and D). Among these subfamilies, ten β-1,3-glucanases were classified, based on amino acid sequences shared considering similarities and uniqueness [1]. The β-1,3 glucanase enzyme is one of three β-glucans found in plants (in addition to β-1,4 glucanases and β-1,3-1,4 glucanases) [2].
Despite being distributed differently among plant organs, glucanases (β-1,3) may play an important role in the physiological systems of plants, including plant growth, seed germination re-production, and fruit ripening [3][4][5]. β-glucans (cellulose, callose, xyloglucan, vmixed-linked glucan—MLG) are cell wall structures, predominant in almost all vegetables. These structures can be degraded by specific enzymes such as β-glucanases [6]. Due to their great potential in plant defense participation, β-1,3 glucanases have been extensively studied, isolated and sequenced [7][8][9][10]. Naturally, β-1,3 glucanase gene expression levels are relatively low, but when a plant–pathogen interaction or elicitors are used, high levels of β-1,3 glucanase can be detected; enzyme accumulation occurs rapidly and consequently hydrolytic activity increases [7][8].
In a fungal invasion, for example, specifically in fungal cell wall degradation through the action of β-1,3 glucanases (Figure 1), oligomers are released, namely β1,3/1,6-D-glucan. These released oligomers can be considered elicitor oligosaccharides. The release of these elicitors induces a plant defense response, demonstrating direct antimicrobial activity [7][8]. Using DNA recombinant technology, a novel β-1,3-glucanase (Gns6) was characterized by functionality. β-1,3-glucanase Gns6 belongs to subfamily A. The gene expression of Gns6 was evaluated at an early stage of rice blast infection, and the involvement of β-1,3-glucanase Gns6 in early plant defense was proved [1]. In order to evaluate the effect of β-1,3-glucanase on the construction of the fungal cell wall, the phytopathogenic fungus was submitted to Gns6 in an antifungal activity bioassay. The results revealed that the enzyme Gns6 exhibited potent antifungal activity against Maganaporthe orzyae, which causes blast disease in rice [1].
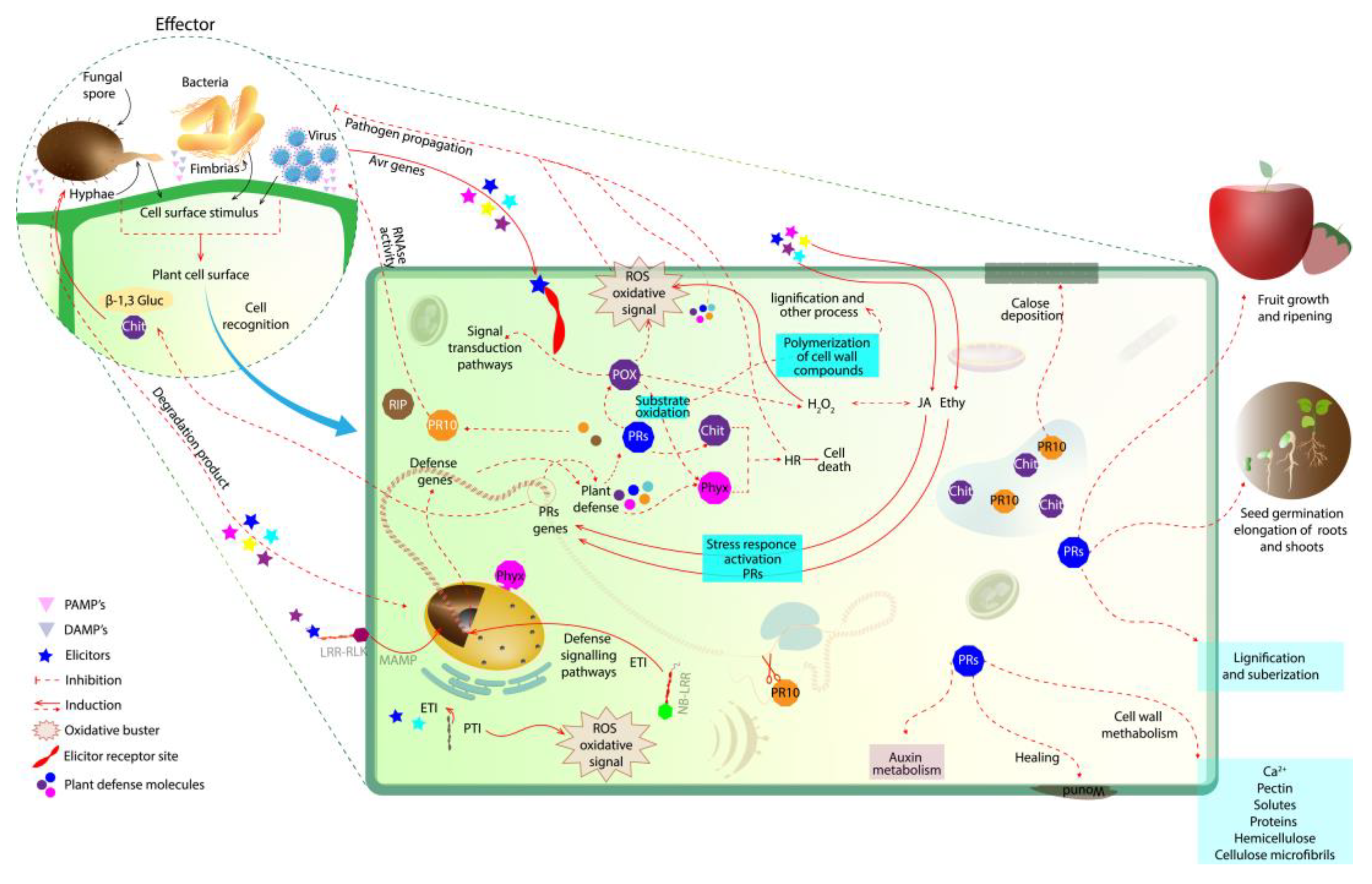
Figure 1. Initial plant defense response in plant–pathogen interaction. At first, the pathogen is recognized on the plant cell wall surface. Then, the elicitors activate a signaling network, where defense genes are activated to produce PR proteins that accumulate and act in the degradation of the pathogenic cell (i.e., β-1,3-gluc and chit; the degradation products of these enzymes can also act as elicitors). The oxidative buster mediates the generation of ROS in an attempt to limit the spread of the pathogen. PRs (i.e., β-1,3-glucanases, chitinases, PRXs, PR10 together with Phyx) are able to induce a hypersensitivity response to prevent the spread of the pathogen to other tissues, releasing elicitors that induce the plant’s defense mechanism. PR10, RBPs and RIPs are also produced by the plant, mediating virus infection. NB-LRR and PR10 act together in gene defense induction. PRX, peroxidase; PRs, pathogen-related proteins; Chit, chitinase; ROS, reactive oxygen species; Phyx, phytoalexin; β-1,3-gluc, β-1,3-glucanases; Ethy, ethylene; JA, jasmonic acid; LRR-RLK, leucine-rich repeat receptor-like protein kinase; NB-LRR, nucleotide-binding and leucine-rich repeat; RBPs, RNA-binding proteins; RIPs, ribosome-inactivating proteins.
Plant β-1,3 glucanases can act in synergism with chitinases, catalyzing the cell wall degradation of microorganisms through the process of hydrolysis of β-1,3 glucans and chitin, respectively (Figure 1). These enzymes are the most studied among the PRs. Furthermore, these enzymes, together with other hydrolases, also participate in the degradation of cell membrane constituents, mainly fungi [6]. Chitinases are enzymes (E.C. 3.2.1.14) belonging to groups 3, 4, 8, and 11 of the PRs.
Chitinases can also act similarly to chitosanases (induced in plants as a response to pathogenic interaction) and are capable of degrading chitosan, which is present in structural components of the cell wall of some species of fungi, including those of the order Mucorales. Chitinases have efficient action in the degradation of chitin, the second-most abundant structural polysaccharide in nature, found in insect exoskeletons; they are also vital components of the fungal cell wall. Additionally, chitinases can be observed in some plant species in response to the action of phytopathogenic viruses [11].
Some chitinases identified so far have demonstrated lysozyme activity, which may also act in bacterial cell wall degradation. This may be antibacterial action, demonstrated by the ability to hydrolyze the β-1,4 bonds that are between N-acetylmuramic acid and N-acetylglucosamine in peptidoglycan-like heterosaccharides present in the cell wall of prokaryotes [12]. As mentioned, during pathogen–plant interactions, elicitor molecules are recognized, and just like β-1,3-glucanase, the chitinases can hydrolyze these elicitors, transforming them into eliciting oligosaccharides (Figure 1). So, the elicitors produced from chitin and β-1,3-glucanase activity can activate a signaling network, where defense genes are activated to produce other PR proteins that accumulate and act in pathogen cell degradation [7][8][13][14].
Functional analysis has revealed that transgenic plants of Arabidopsis, overexpressing the endochitinase gene, proved to be resistant to Xanthomonas campestris pv. campestris (Xcc) when compared to wild-type plants. This endochitinase was identified in cabbage plants and showed up-regulation 24 h after infection Xcc. Gene expression analysis showed high levels of the endochitinase gene when compared to the uninoculated cabbage plant [15]. The analysis of Cucumis sativus L. showed the induction of genes encoding chitinase in plant roots during infection by Fusarium oxysporum f. sp. cucumerinum (Foc) [16].
An in vitro assay with purified chitinases Chi2 and Chi14 showed that proteins limited Foc growth. In addition, the gene silencing of Chi14, using the technique of virus-induced gene silencing (VIGS), increased the plant’s sensitivity to fungus. Chi2 gene silencing drastically compromised the activation of the jasmonic acid pathway gene, which is a phytohormone important in plant defense signaling. These results corroborate the hypothesis that chitinase (Chi2) may play a key role in plant resistance [16]. The overexpression of type II chitinase (LcCHI2) in Leymus chinensis conferred increased hydrolytic activity in transgenic tobacco and corn plants, which have been shown to be more resistant to pathogens and salt stress [17].
Twenty-six chitinase genes were identified in Morus notablis plants [18]. The differential expression of one of these enzymes, MnChi18, leads to an increased defense against Botrytis cinera. The plant models overexpressing MnChi18 were protected from damage and were shown to be involved in B. cinera resistance [18]. Finally, another study showed that the chitinase gene can positively regulate the hypersensitive and defense responses of Capsicum annuum L. to infection caused by Colletotrichum acutatum [19].
In summary, both β-1,3-glucanase and chitinase have been shown to play a significant role in plant defense against microbial agents. It is known that β-1,3-glucanases accumulate during pathogen attack and can act in the hydrolysis of the pathogen cell wall. As mentioned above, the substrate for this enzyme, β-1,3-glucans, can be found in several microorganisms [1]. Faced with the action of β-1,3-glucanases and chitinase enzymes, oligomers are released, which are β1,3/1,6-D-glucano and chitin, respectively. These released oligomers can be called elicitor oligosaccharides. Elicitor release induces a plant defense response. The activity of both enzymes can cause the depolymerization of structural saccharides present in the pathogen wall, degrading it [20].
2. PR-9 Family: Peroxidases
Together, plant peroxidases, β-1,3-glucanases and chitinases act in the early plant infection stages [21]. Once the plant has detected pathogen elicitors or abiotic stress, a series of events, such as oxidative burst, takes place in an attempt to protect the plant from damage induced by ROS. Plant ROS production leads to oxidative burst (Figure 1). This action plays an important role in direct defense by promoting lignification and pathogen intoxication due to ROS accumulation [21][22][23].
Currently, the peroxidases are classified into two groups, the first of which is nonheme peroxidase, which is found in prokaryotes and eukaryotes (including halo-peroxidases, NADH peroxidases, thiol peroxidases, and alkylhydro-peroxidase). The second group, heme-peroxidases, is composed of two superfamilies: (1) the peroxidase-cyclooxygenase superfamily (PCOXS) and (2) the peroxidase-catalase superfamily (PCATS). The PCOXS representatives are known as the animal-peroxidase superfamily, while the PCATS are commonly called the nonanimal heme peroxidases [24][25]. Nowadays, three classes are found in the nonanimal peroxidases: class I (ascorbate peroxidase, yeast cytochrome and bacterial catalase peroxidases), class II (heme peroxidase, includes lignin peroxidase, manganese peroxidase and versatile peroxidase) and class III (found in plants). The class III peroxidases correspond to about 70% of plant-derived peroxidases [25]. Plant peroxidases (POX, EC 1.11.1.7) are antioxidant enzymes, belonging to group 9 of the PRs (PR-9) [26].
Plant peroxidases have an important role in plant physiology (Figure 1), including lignification and wound healing; these enzymes can also participate in the regulation of cell elongation [27]. Peroxidases play a plant defense role against pathogens. Besides participating in cell signaling after infection, peroxidases can polymerize macromolecules which, after being deposited on the extracellular surface, can promote cell wall strengthening and thus make pathogen invasion more difficult. Peroxidases can also induce the oxidative degradation of phenolic compounds in the cell rupture region caused by pathogens in the first infection stages [27]. Along with two other oxidizing enzyme families (unrelated), the laccases (LACs) and the polyphenol oxidases (PPOs) family, the PRXs make up the phenoloxidases. PRXs can oxidize substrates, including some phenols, through the reduction of H2O2 or organic peroxides [27][28][29]. Some phenols can generate oxygen radicals, which can be extremely reactive and harmful to the plant. PRXs and other phenoloxidases play a protective role, leading to the oxidative degradation of some phenol forms at the site of infection [29]. Thus, the use of plant peroxidases arouses great industrial interest as potential biodegradable agents. PRX from plants can degrade residual phenols in water, from industrial wastewater [30][31][32].
Diverse isoforms of the peroxidase family are found throughout the plant and are capable of oxidizing numerous molecules. POXs are involved in many biological activities (Figure 1), such as cellular detoxification and the elimination of ROS (including 1 O2, singlet oxygen; O2•−, superoxide anion; H2O2, hydrogen peroxide; and OH•, hydroxyl radical). Peroxidases are important enzymes for maintaining the redox homeostasis of plant cells [33].
In addition, in the first moment of plant stress, ROS are produced to protect the plant from oxidative stress, since the oxidative burst can be lethal to the plant. The balance between antioxidant (AOX) and ROS production is necessary for plant normality. Plant detoxification is also needed, and this can be through peroxidase and other enzymes, such as catalase, superoxide dismutase, etc. [34]. An imbalance between AOX and ROS, either due to AOX depletion or ROS excess, can prolong oxidative stress, which can compromise the production of lipids, amino acids, proteins, nucleotide acid and pigments [35]. The remaining oxidative stress also causes cellular damage, leading to membrane injury, organelle function losses, reduced metabolic efficiency, reduced carbon fixation, electrolyte leakage, and chromatid breaks and mutation. All this damage can lead to growth reduction, yield loss and cell death. Peroxidase action is essential for maintaining cellular balance [35].
Both peroxidase and NADPH oxidase, present in the plant cell wall, play an important role in the apoplastic oxidative burst after microbial attack against plants. In Arabidopsis, after interaction with the fungus Alternaria brassicola, cell wall peroxidases (named PRX33 and PRX34) and NADPH oxidase mediated the oxidative burst in the plant. These enzymes are considered to be the main catalyst of the oxidative burst process [36]. A characterization study also demonstrated that the Arabidopsis mutant prx34 can reduce ROS and callose accumulation after Flg22-elicitor treatment. These results corroborate other findings, showing that the PRX34 enzyme could be an important component for plant disease resistance [37].
Another work overexpressed a peroxidase (swpa4) gene, and the stress-related functions of these enzymes in Ipomoea batatas L. were evaluated. The results indicated that swapa4 gene overexpression can protect the plant from damage [38]. Furthermore, these results suggested that transgenic sweet potato, overexpressing the PRX genes, can respond more efficiently to saline stress [38]. An in vivo bioassay evaluation, using transgenic Arabidopsis, showed that lines overexpressing PRX genes (cotton gene GhPRXIIB) were capable of tolerating and limiting nematode infection [39].
Some POX enzymes, such as ascorbate peroxidase (APX) and glutathione peroxidase (GPx), can catalyze the conversion of H2O2 to H2O [40][41]. Many APX isoforms can be found in different subcellular compartments, including chloroplasts, mitochondria, peroxisome and cytosol [42], and can play an important role in oxidative defense metabolism [43][44]. Transgenic plants overexpressing glutathione peroxidase (named AtGPXL5) revealed that this enzyme gene can participate in ethylene (ET) biosynthesis and signaling [45].
After treatment with the ET-precursor (1-aminocyclopropane-1-carboxylic acid—ACC), transgenic plants show glutathione- and thioredoxin-induced activity and other enzymes involved in ROS processing, which suggests the involvement of the AtGPXL5 gene with ethylene signaling and thus also with plant cell defense [45]. Transgenic plants of Citrus sinensis, overexpressing the CsPrx25 gene and encoding a class III peroxidase, show ROS homeostasis and increased H2O2 levels and consequently a strong hypersensitivity reaction to Xcc. The results also show that CsPrx25 gene overexpression contributes to the lignification process of the cell wall, increasing plant resistance [46].
In summary, ROS production increases, such as H2O2, and seems to protect the plant against environmental stimulus including pathogens, but can cause significant stress, since ROS accumulation can lead to cellular toxicity. APX and GPx can act in cellular homeostasis under oxidative stress, protecting the plant [41][47].
3. PR-10 Family: Ribonucleases
As mentioned, the ribonucleases (RNase) are a member of group 10 of PRs. They show approximately 17 kDa and exhibit a hydrophobic core capable of binding a wide variety of ligands. Ribonuclease PR10 has demonstrated ligand ability for low-molecular-mass compounds. The PR-10 hydrophobic cavity can bind with small molecules, and this hydrophobic cavity can be considered as a general feature of such enzymes [48]. Many studies have reported the protein–ligand interaction of PR-10 proteins, as reviewed by [48]. These enzymes can bind to steroids, cytokinin, flavonoids and fatty acids, phytoprostanes, phytomelatonin, gibberellic acid and plant metabolites, with molecules involved in flavor production and color. Ribonuclease enzymes can interact with phytohormones in the hormone-mediated signaling process [48][49].
Homologs share this conserved structure, but function is not a universal characteristic among the members of the group. These enzymes have been identified in different plant species. However, no unique biological function has been assigned to PR-10 proteins. Among the functions assigned to PR-10 are plant growth and development, as well as antioxidation, UV protection, and pathogen defense. An unusual protein was found in rubber trees and presented activity like the PR-10 class. After structural characterization, plant protection against the Rigidoporus microporus fungus was related to this protein. The structural analysis demonstrated that these proteins can bind with a deoxycholic acid ligand [50]. Deoxycholic acid is a bile acid (bile acid deoxycholic acid—DCA), which demonstrated action related to plant defense response. DCA can induce defense in Arabidopsis plants and reduce bacterial proliferation [51]. It is possible to observe the up-regulation of PR10 enzymes during plant pathogen interaction and/or direct induction after applying external phytohormones, proving the protective action of PR10 during plant–pathogen interaction [49][52][53]. Additionally, it is possible to observe an increase in PR10 abundance during interactions caused by viruses and fungi [54][55][56][57][58].
Besides PR-10′s involvement in the signaling pathways of defense genes, ribonucleolytic activity to cleave invading pathogens has been reported (Figure 1), causing the pathogen’s RNA cleavage [59]. During pathogen infection, the RNase activity of PR10 proteins can cause a cytotoxic cell impact and inhibit pathogen growth, degrading the pathogen cell [54][55][56][57][58]. This inhibition occurs mainly through ribonuclease penetration into the pathogen, with PR10 phosphorylation subsequently occurring, and consequently the destruction of pathogenic cell RNAs [60].
RNase activity can be exhibited by several PR-10 proteins but it is not believed to be a universal characteristic [61]. RNase activity is required under biotic and abiotic stress, since these proteins are involved in plant HR signalization, in programmed cell death control and/or apoptosis process [62][63]. Much evidence has been reported on the general activity of PR-10 against different phytopathogens such as fungi, bacteria, and viruses [48][59]. Additionally, one report indicated the protease inhibitory activity of PR-10 in the root-knot nematode Meloidogyne incognita [64].
Concerning the PR-10 activity against pathogens, although not well-explained, these enzymes are believed to be related to the endogenous cytokinin (CK) concentrations and CK in negative feedback regulation. These cytokines are involved in plant immunity modulation, acting directly in the plant defense response to many pathogens [53][59][65].
In addition, PR-10 proteins can interact with plant hormones such as ABA, JA, auxins, ethylene, and SA, which are involved in hormone-mediated signaling to mitigate damage suffered by the plant, caused by biotic and abiotic stress [59][66]. In plants infected with Verticillium dahlia, PR10 genes were found to be up-regulated after an expression profile investigation in leaves, roots and stems of strawberry plants [52]. Once again, the induction of some phytohormones, including ABA, SA, JA, and gibberellic acid, was seen in the early stages of plant–pathogen interaction. In roots, just two hormones were induced, indole acetic acid (IAA) and JA, but in the late stages of infection [52].
In Valsa mali fungus, VmP1 is a virulence factor and can interact with PR10 (named MdPR10) in vivo. The MdPR10 gene present in Malus domestica is a VmP1 target, and when the silencing of the MdPR10 gene occurs, plant susceptibility increases in plants, while gene overexpression decreases the infection, showing the role of MdPR10 in plant defense against V. mali [67]. Mahmoud et al. (2020) [68] demonstrated, with exogenous products to induce the plant’s systemic resistance, that after treatment the plants showed increased abundance of PR10 as well as another protein, which is also used as a marker of systemic acquired resistance (SAR), namely, phenylalanine ammonia lyase (PAL). The in vivo results demonstrate antiviral activity against the tomato pathogen Tobacco Mosaic Virus (TMV) [68].
This entry is adapted from the peer-reviewed paper 10.3390/plants12112226
References
- Wang, Y.; Liu, M.; Wang, X.; Zhong, L.; Shi, G.; Xu, Y.; Li, Y.; Li, R.; Huang, Y.; Ye, X.; et al. A novel β-1,3-glucanase Gns6 from rice possesses antifungal activity against Magnaporthe oryzae. J. Plant Physiol. 2021, 265, 153493.
- Linton, S.M. The structure and function of cellulase (endo-β-1, 4-glucanase) and hemicellulase (β-1, 3-glucanase and endo-β-1, 4-mannase) enzymes in invertebrates that consume materials ranging from microbes, algae to leaf litter. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2020, 240, 110354.
- Balasubramanian, V.; Vashisht, D.; Cletus, J.; Sakthivel, N. Plant β-1, 3-glucanases: Their biological functions and transgenic expression against phytopathogenic fungi. Biotechnol. Lett. 2012, 34, 1983–1990.
- De Storme, N.; Geelen, D. Callose homeostasis at plasmodesmata: Molecular regulators and developmental relevance. Front. Plant Sci. 2014, 5, 138.
- Garcia-Gimenez, G.; Russell, J.; Aubert, M.K.; Fincher, G.B.; Burton, R.A.; Waugh, R.; Tucker, M.R.; Houston, K. Barley grain (1, 3; 1, 4)-β-glucan content: Effects of transcript and sequence variation in genes encoding the corresponding synthase and endohydrolase enzymes. Sci. Rep. 2019, 9, 17250.
- Perrot, T.; Pauly, M.; Ramírez, V. Emerging Roles of β-Glucanases in Plant Development and Adaptative Responses. Plants 2022, 11, 1119.
- Finkina, E.I.; Melnikova, D.N.; Bogdanov, I.V.; Ovchinnikova, T.V. Plant pathogenesis-related proteins PR-10 and PR-14 as components of innate immunity system and ubiquitous allergens. Curr. Med. Chem. 2017, 24, 1772–1787.
- Ali, Z.; Serag, M.; Demirer, G.; Torre, B.; di Fabrizio, E.; Landry, M.; Habuchi, S.; Mahfouz, M. The DNA–carbon nanotube binding mode determines the efficiency of carbon nanotube-mediated DNA delivery to intact plants. ACS Appl. Nano Mater. 2021, 5, 4663–4676.
- Paniagua, C.; Perry, L.; Benitez-Alfonso, Y. A phylogenetic and transcriptomic study of the β-1, 3-glucanase family in tomato identifies candidate targets for fruit improvement. bioRxiv 2021.
- Wu, Q.; Dou, X.; Wang, Q.; Guan, Z.; Cai, Y.; Liao, X. Isolation of β-1,3-Glucanase-Producing Microorganisms from Poria cocos Cultivation Soil via Molecular Biology. Molecules 2018, 23, 1555.
- Pusztahelyi, T. Chitin and chitin-related compounds in plant–fungal interactions. Mycology 2018, 9, 189–201.
- Wanke, A.; Malisic, M.; Wawra, S.; Zuccaro, A. Unraveling the sugar code: The role of microbial extracellular glycans in plant–microbe interactions. J. Exp. Bot. 2021, 72, 15–35.
- Héloir, M.-C.; Adrian, M.; Brulé, D.; Claverie, J.; Cordelier, S.; Daire, X.; Dorey, S.; Gauthier, A.; Lemaître-Guillier, C.; Negrel, J.; et al. Recognition of Elicitors in Grapevine: From MAMP and DAMP Perception to Induced Resistance. Front. Plant Sci. 2019, 10, 1117.
- Jamiołkowska, A. Natural Compounds as Elicitors of Plant Resistance Against Diseases and New Biocontrol Strategies. Agronomy 2020, 10, 173.
- Santos, C.; Nogueira, F.C.S.; Domont, G.B.; Fontes, W.; Prado, G.S.; Habibi, P.; Santos, V.O.; Oliveira-Neto, O.B.; Grossi-de-Sá, M.F.; Jorrín-Novo, J.V.; et al. Proteomic analysis and functional validation of a Brassica oleracea endochitinase involved in resistance to Xanthomonas campestris. Front. Plant Sci. 2019, 10, 414.
- Xu, J.; Zhang, N.; Wang, K.; Xian, Q.; Dong, J.; Qi, X.; Chen, X. Chitinase Chi 2 Positively Regulates Cucumber Resistance against Fusarium oxysporum f. sp. cucumerinum. Genes 2022, 13, 62.
- Liu, X.; Yu, Y.; Liu, Q.; Deng, S.; Jin, X.; Yin, Y.; Guo, J.; Li, N.; Liu, Y.; Han, S.; et al. A Na2CO3-Responsive Chitinase Gene From Leymus chinensis Improve Pathogen Resistance and Saline-Alkali Stress Tolerance in Transgenic Tobacco and Maize. Front. Plant Sci. 2020, 11, 1–12.
- Xin, Y.; Wang, D.; Han, S.; Li, S.; Gong, N.; Fan, Y.; Ji, X. Characterization of the chitinase gene family in mulberry (Morus notabilis) and MnChi18 involved in resistance to Botrytis cinerea. Genes 2021, 13, 98.
- Ali, M.; Li, Q.H.; Zou, T.; Wei, A.M.; Gombojab, G.; Lu, G.; Gong, Z.H. Chitinase Gene Positively Regulates Hypersensitive and Defense Responses of Pepper to Colletotrichum acutatum Infection. Int. J. Mol. Sci. 2020, 21, 6624.
- Franzener, G.; Schwan-Estrada, K.R.F.; Moura, G.S.; Kuhn, O.J.; Stangarlin, J.R. Induction of defense enzymes and control of anthracnose in cucumber by Corymbia citriodora aqueous extract. Summa Phytopathol. 2018, 44, 10–16.
- Oliveira, M.D.M.; Varanda, C.M.R.; Félix, M.R.F. Induced resistance during the interaction pathogen x plant and the use of resistance inducers. Phytochem. Lett. 2016, 15, 152–158.
- Choudhary, A.; Kumar, A.; Kaur, N. ROS and oxidative burst: Roots in plant development. Plant Divers. 2020, 42, 33–43.
- Sorokan, A.V.; Burhanova, G.F.; Maksimov, I.V. Anionic peroxidase-mediated oxidative burst requirement for jasmonic acid-dependent Solanum tuberosum defence against Phytophthora infestans. Plant Pathol. 2018, 67, 349–357.
- Bela, K.; Riyazuddin, R.; Csiszár, J. Plant Glutathione Peroxidases: Non-Heme Peroxidases with Large Functional Flexibility as a Core Component of ROS-Processing Mechanisms and Signalling. Antioxidants 2022, 11, 1624.
- Twala, P.P.; Mitema, A.; Baburam, C.; Feto, N.A. Breakthroughs in the discovery and use of different peroxidase isoforms of microbial origin. AIMS Microbiol. 2020, 6, 330–349.
- Kaur, A.; Pati, P.K.; Pati, A.M.; Nagpal, A.K. Physico-chemical characterization and topological analysis of pathogenesis-related proteins from Arabidopsis thaliana and Oryza sativa using in-silico approaches. PLoS ONE 2020, 15, e0239836.
- Kaur, S.; Samota, M.K.; Choudhary, M.; Choudhary, M.; Pandey, A.K.; Sharma, A.; Thakur, J. How do plants defend themselves against pathogens-Biochemical mechanisms and genetic interventions. Physiol. Mol. Biol. Plants 2022, 28, 485–504.
- Blaschek, L.; Pesquet, E. Phenoloxidases in plants—How structural diversity enables functional specificity. Front. Plant Sci. 2021, 12, 754601.
- Campos, Â.D.; Ferreira, A.G.; Hampe, M.M.V.; Antunes, I.F.; Brancão, N.; Silveira, E.P.d.; Osório, V.A.; Augustin, E. Peroxidase and polyphenol oxidase activity in bean anthracnose resistance. Pesqui. Agropecu. Bras. 2004, 39, 637–643.
- Basumatary, D.; Yadav, H.S.; Yadav, M. The role of peroxidases in the bioremediation of organic pollutants. Nat. Prod. J. 2023, 13, 60–77.
- Kurnik, K.; Treder, K.; Skorupa-Kłaput, M.; Tretyn, A.; Tyburski, J. Removal of phenol from synthetic and industrial wastewater by potato pulp peroxidases. Water Air Soil Pollut. 2015, 226, 254.
- Lakshmi, S.; Shashidhara, G.M.; Madhu, G.M.; Muthappa, R.; Vivek, H.K.; Nagendra Prasad, M.N. Characterization of peroxidase enzyme and detoxification of phenols using peroxidase enzyme obtained from Zea mays L waste. Appl. Water Sci. 2018, 8, 207.
- Sellami, K.; Couvert, A.; Nasrallah, N.; Maachi, R.; Abouseoud, M.; Amrane, A. Peroxidase enzymes as green catalysts for bioremediation and biotechnological applications: A review. Sci. Total Environ. 2022, 806, 150500.
- Zechmann, B. Subcellular Roles of Glutathione in Mediating Plant Defense during Biotic Stress. Plants 2020, 9, 1067.
- Hasanuzzaman, M.; Nahar, K.; Hossain, M.S.; Mahmud, J.A.; Rahman, A.; Inafuku, M.; Oku, H.; Fujita, M. Coordinated Actions of Glyoxalase and Antioxidant Defense Systems in Conferring Abiotic Stress Tolerance in Plants. Int. J. Mol. Sci. 2017, 18, 200.
- Kámán-Tóth, E.; Dankó, T.; Gullner, G.; Bozsó, Z.; Palkovics, L.; Pogány, M. Contribution of cell wall peroxidase-and NADPH oxidase-derived reactive oxygen species to Alternaria brassicicola-induced oxidative burst in Arabidopsis. Mol. Plant Pathol. 2019, 20, 485–499.
- Zhao, L.; Phuong, L.T.; Luan, M.T.; Fitrianti, A.N.; Matsui, H.; Nakagami, H.; Noutoshi, Y.; Yamamoto, M.; Ichinose, Y.; Shiraishi, T. A class III peroxidase PRX34 is a component of disease resistance in Arabidopsis. J. Gen. Plant Pathol. 2019, 85, 405–412.
- Kim, Y.-H.; Kim, H.S.; Park, S.-C.; Ji, C.Y.; Yang, J.W.; Lee, H.U.; Kwak, S.-S. Overexpression of swpa4 peroxidase enhances tolerance to hydrogen peroxide and high salinity-mediated oxidative stress in transgenic sweetpotato plants. Plant Biotechnol. Rep. 2020, 14, 301–307.
- dos Santos, C.; Carmo, L.S.T.; Távora, F.T.P.K.; Lima, R.F.C.; Da Nobrega Mendes, P.; Labuto, L.B.D.; De Sá, M.E.L.; Grossi-De-Sa, M.F.; Mehta, A. Overexpression of cotton genes GhDIR4 and GhPRXIIB in Arabidopsis thaliana improves plant resistance to root-knot nematode (Meloidogyne incognita) infection. 3 Biotech 2022, 12, 211.
- Do, J.H.; Park, S.Y.; Park, S.H.; Kim, H.M.; Ma, S.H.; Mai, T.D.; Shim, J.S.; Joung, Y.H. Development of a genome-edited tomato with high ascorbate content during later stage of fruit ripening through mutation of SlAPX4. Front. Plant Sci. 2022, 13, 1–11.
- Pandey, S.; Fartyal, D.; Agarwal, A.; Shukla, T.; James, D.; Kaul, T.; Negi, Y.K.; Arora, S.; Reddy, M.K. Abiotic stress tolerance in plants: Myriad roles of ascorbate peroxidase. Front. Plant Sci. 2017, 8, 581.
- Wu, B.; Wang, B. Comparative analysis of ascorbate peroxidases (APXs) from selected plants with a special focus on Oryza sativa employing public databases. PLoS ONE 2019, 14, e0226543.
- Akram, N.A.; Shafiq, F.; Ashraf, M. Ascorbic acid-a potential oxidant scavenger and its role in plant development and abiotic stress tolerance. Front. Plant Sci. 2017, 8, 613.
- Chin, D.-C.; Kumar, R.S.; Suen, C.-S.; Chien, C.-Y.; Hwang, M.-J.; Hsu, C.-H.; Xuhan, X.; Lai, Z.X.; Yeh, K.-W. Plant cytosolic ascorbate peroxidase with dual catalytic activity modulates abiotic stress tolerances. Iscience 2019, 16, 31–49.
- Riyazuddin, R.; Bela, K.; Poór, P.; Szepesi, Á.; Horváth, E.; Rigó, G.; Szabados, L.; Fehér, A.; Csiszár, J. Crosstalk between the Arabidopsis glutathione peroxidase-like 5 Isoenzyme (AtGPXL5) and ethylene. Int. J. Mol. Sci. 2022, 23, 5749.
- Li, Q.; Qin, X.; Qi, J.; Dou, W.; Dunand, C.; Chen, S.; He, Y. CsPrx25, a class III peroxidase in Citrus sinensis, confers resistance to citrus bacterial canker through the maintenance of ROS homeostasis and cell wall lignification. Hortic. Res. 2020, 7, 192.
- Kuo, E.Y.; Cai, M.-S.; Lee, T.-M. Ascorbate peroxidase 4 plays a role in the tolerance of Chlamydomonas reinhardtii to photo-oxidative stress. Sci. Rep. 2020, 10, 13287.
- Aglas, L.; Soh, W.T.; Kraiem, A.; Wenger, M.; Brandstetter, H.; Ferreira, F. Ligand binding of PR-10 proteins with a particular focus on the Bet v 1 allergen family. Curr. Allergy Asthma Rep. 2020, 20, 25.
- Ghorbel, M.; Zribi, I.; Missaoui, K.; Drira-Fakhfekh, M.; Azzouzi, B.; Brini, F. Differential regulation of the durum wheat Pathogenesis-related protein (PR1) by Calmodulin TdCaM1. 3 protein. Mol. Biol. Rep. 2021, 48, 347–362.
- Longsaward, R.; Pengnoo, A.; Kongsawadworakul, P.; Viboonjun, U. A novel rubber tree PR-10 protein involved in host-defense response against the white root rot fungus Rigidoporus microporus. BMC Plant Biol. 2023, 23, 157.
- Zarattini, M.; Launay, A.; Farjad, M.; Wénès, E.; Taconnat, L.; Boutet, S.; Bernacchia, G.; Fagard, M. The bile acid deoxycholate elicits defences in Arabidopsis and reduces bacterial infection. Mol. Plant Pathol. 2017, 18, 540–554.
- Besbes, F.; Habegger, R.; Schwab, W. Induction of PR-10 genes and metabolites in strawberry plants in response to Verticillium dahliae infection. BMC Plant Biol. 2019, 19, 128.
- Genzel, F.; Franken, P.; Witzel, K.; Grosch, R. Systemic induction of salicylic acid-related plant defences in potato in response to Rhizoctonia solani AG 3 PT. Plant Pathol. 2018, 67, 337–348.
- Jain, D.; Khurana, J.P. Role of Pathogenesis-Related (PR) Proteins in Plant Defense Mechanism. In Molecular Aspects of Plant-Pathogen Interaction; Singh, A., Singh, I.K., Eds.; Springer: Singapore, 2018; pp. 265–281.
- Besbes, F.; Franz-Oberdorf, K.; Schwab, W. Phosphorylation-dependent ribonuclease activity of Fra a 1 proteins. J. Plant Physiol. 2019, 233, 1–11.
- Huh, S.U.; Paek, K.-H. Plant RNA binding proteins for control of RNA virus infection. Front. Physiol. 2013, 4, 397.
- Islam, M.M.; Qi, S.; Zhang, S.; Amin, B.; Yadav, V.; El-Sappah, A.H.; Zhang, F.; Liang, Y. Genome-Wide Identification and Functions against Tomato Spotted Wilt Tospovirus of PR-10 in Solanum lycopersicum. Int. J. Mol. Sci. 2022, 23, 1502.
- Wieczorek, P.; Wrzesińska, B.; Frąckowiak, P.; Przybylska, A.; Obrępalska-Stęplowska, A. Contribution of Tomato torrado virus Vp26 coat protein subunit to systemic necrosis induction and virus infectivity in Solanum lycopersicum. Virol. J. 2019, 16, 9.
- Sinha, R.K.; Verma, S.S.; Rastogi, A. Role of Pathogen-Related Protein 10 (PR 10) under abiotic and biotic stresses in plants. Phyton 2020, 89, 167.
- Kaur, A.; Kaur, S.; Kaur, A.; Sarao, N.K.; Sharma, D. Pathogenesis-Related Proteins and Their Transgenic Expression for Developing Disease-Resistant Crops: Strategies Progress and Challenges. In Plant Breeding - New Perspectives; IntechOpen: London, UK, 2022; pp. 6–8.
- McBride, J.K.; Cheng, H.; Maleki, S.J.; Hurlburt, B.K. Purification and characterization of pathogenesis related class 10 panallergens. Foods 2019, 8, 609.
- Choi, D.S.; Hwang, I.S.; Hwang, B.K. Requirement of the cytosolic interaction between pathogenesis-related protein 10 and leucine-rich repear protein 1 for cell death and defense signaling in pepper. Plant Cell 2012, 24, 1675–1690.
- Lee, O.R.; Pulla, R.K.; Kim, Y.J.; Balusamy, S.R.; Yang, D.C. Expression and stress tolerance of PR10 genes from Panax ginseng C. A. Meyer. Mol. Biol. Rep. 2012, 39, 2365–2374.
- Andrade, L.B.d.S.; Oliveira, A.S.; Ribeiro, J.K.C.; Kiyota, S.; Vasconcelos, I.M.; de Oliveira, J.T.A.; de Sales, M.P. Effects of a novel pathogenesis-related class 10 (PR-10) protein from crotalaria pallida roots with papain inhibitory activity against root-knot nematode Meloidogyne incognita. J. Agric. Food Chem. 2010, 58, 4145–4152.
- Li, B.; Wang, R.; Wang, S.; Zhang, J.; Chang, L. Diversified regulation of cytokinin levels and signaling during Botrytis cinerea infection in Arabidopsis. Front. Plant Sci. 2021, 12, 584042.
- Gao, L. Structure Analysis of a Pathogenesis-Related 10 Protein from Gardenia jasminoides. IOP Conf. Ser. Earth Environ. Sci. 2019, 2019, 042005.
- Wang, W.; Nie, J.; Lv, L.; Gong, W.; Wang, S.; Yang, M.; Xu, L.; Li, M.; Du, H.; Huang, L. A Valsa mali Effector Protein 1 Targets Apple (Malus domestica) Pathogenesis-Related 10 Protein to Promote Virulence. Front. Plant Sci. 2021, 12, 1–12.
- Mahmoud, F.; Bondok, A.; Khalifa, W.; Abou Abbas, F. Enhancing Tomato Plant Resistance Against Tobacco Mosaic Virus Using Riboflavin. Arab Univ. J. Agric. Sci. 2020, 28, 1257–1270.
This entry is offline, you can click here to edit this entry!
