Periradicular tissues have a rich supply of peripheral afferent neurons, also known as nociceptive neurons, originating from the trigeminal nerve. While their primary function is to relay pain signals to the brain, these are known to be involved in modulating innate and adaptive immunity by initiating neurogenic inflammation (NI).
1. Introduction
Apical periodontitis (AP) is an inflammatory disease of periradicular tissue caused by oral opportunistic bacteria and their toxins retained in the apical regions of the periodontium [
1,
2]. Rich in blood vessels and nerve fibers, the periradicular tissue is composed of fibroblasts, epithelial cells, odontoblasts, cementoblasts, osteoblasts, osteoclasts, macrophages, and undifferentiated stem cells that form the periodontal ligament (PDL) and alveolar bone [
3,
4]. An infected root canal system in the apical periodontium causes all these immune cells and precursor cells to interact with one another, thereby eliciting an immune response against pathogens and pathogen-associated molecular patterns (PAMPs) [
5]. However, AP can persist asymptomatically, without common clinical symptoms such as pain and swelling, while afferent sensory neurons that typically relay pain continue to engage in immune responses at the molecular level [
6,
7].
Neurogenic inflammation (NI) is set in motion when neuropeptides such as Substance P (SP) and Calcitonin Gene-Related Peptide (CGRP), among others, released by activated nociceptors act on resident immune cells and endothelial cells, causing vascular changes resulting in an influx of migrating immune cells to an inflamed tissue. To facilitate their function in NI, endothelial cells (vascular cells), mast cells, dendritic cells, T and B lymphocytes, and macrophages express cell-surface receptors for neuropeptides released by nociceptors [
8,
9]. Neuropeptides activate immune cells which release proinflammatory cytokines such as tumor necrosis factor (TNF)-α, interleukin (IL)-1β, IL-6, nerve growth factor (NGF), and prostaglandin E2 (PGE2) that are in turn known to activate sensory neurons [
10,
11]. Neuropeptides also play a role in regulating anti-inflammatory roles of immune cells. For example, CGRP has been shown to inhibit the production of pro-inflammatory cytokines and enhance the expression of a regulatory phenotype in activated macrophages [
12]. This reflects on the pleiotropic nature of neuropeptides that are capable of modulating immune response.
2. Macrophages in Apical Periodontitis
Inflammation is the physiological response to injury, stress, or infection. In terms of the immune cells involved, inflammation is the state in which innate and adaptive cells are activated, undergo differentiation, and migrate to resolve inflammation while restoring tissue homeostasis. A rapid host immune response is capable of resolving acute inflammation. On the other hand, prolonged exposure to triggers such as damage-associated molecular patterns (DAMPs), PAMPS, and pathogens results in a state of chronic inflammation that sees different immune and non-immune cells in action [
15].
Acute AP is marked by the presence of periapical abscesses, while periapical granulomas are typically a sign of chronic AP, typically diagnosed via the radiographic visualization of rarefying osteitis in periapical tissues. AP is dynamic in nature because of the different immune cell types participating in different stages of AP. This dynamic nature can be observed via the progressive change in the distribution of immune cells in the periapical inflammatory infiltrate [
16,
17]. Immune cells can also undergo polarization to different phenotypes in order to facilitate the host immune response in disease or wound-healing [
18,
19].
Physiologically, the PDL has a key role in responding to microbes located within the pulp space. The PDL is a highly vascularized tissue, with up to ten times higher vascular volume than other fibrous connective tissues [
20]. The blood vessels in PDL primarily participate in tooth support and shock absorption [
21]. The activation of immune cells such as macrophages leads to the release of proinflammatory cytokines that increase the expression of cellular adhesion molecule (CAM-1) in endothelial cells, thereby promoting the local adhesion of monocytes and leukocytes [
18,
22]. This local response, combined with vasodilation caused by initial inflammation response, leads to the active infiltration of monocytes to the site of inflammation, where growth factors, pro-inflammatory cytokines, and microbial products induce cellular differentiation [
23,
24]. Once at the site of inflammation, these circulatory monocytes can be differentiated into macrophages or dendritic cells [
25].
Toll-like receptor-4 (TLR4) is a sensory receptor predominantly expressed by human myeloid cells (monocytes and granulocytes) that recognize PAMPs such as lipopolysaccharides (LPS) expressed by Gram-negative bacteria [
26]. Activated TLR4 recruits adaptor proteins that undergo phosphorylation. Myeloid differentiation primary response 88 (MyD88), Interleukin-1 receptor-associated kinase (IRAK), and tumor necrosis factor receptor-associated factor-6 (TRAF-6) are examples of such adaptor proteins. Phosphorylated adaptor proteins activate Nuclear Factor kappa B (NFκB) and mitogen-activated protein kinase (MAPK) signaling pathways, resulting in the expression of pro-inflammatory cytokines [
27].
Macrophages are known to play an active role in resolving inflammation [
29]. Activated macrophages can polarize into functional phenotypes of classically activated M1, or alternatively activated M2, to bring about pro- and anti-inflammatory effects, respectively. M1 macrophages produce pro-inflammatory cytokines TNF-α, IL-1β, and IL-6, which stimulate the production of tissue-damaging proteases and prostaglandins while mediating bone resorption. M1 macrophages also produce chemokines such as C-X-C motif chemokine ligand (CXCL)9 and CXCL10 that attract members of the adaptive immunity to the site of inflammation [
30,
31]. M2 macrophages are known to produce anti-inflammatory cytokines Transforming Growth Factor (TGF)-β and IL-10, which downregulate inflammation and immune response [
32,
33]. Their phagocytic capabilities help clear apoptotic cells and debris in healing tissues [
5,
34].
3. Role of Macrophages in Alveolar Bone Resorption
Resident macrophages in periodontal tissues secrete inflammatory cytokines and chemokines in response to pathogens. Qualitative and quantitative analyses have identified macrophages as the major constituent immune cell type in periapical granulomas [
35]. This can be partly attributed to the vasodilation caused by the initial immune response, which in turn leads to the active infiltration of monocytes to the site of inflammation, wherein the growth factors, pro-inflammatory cytokines, and microbial products would induce differentiation to macrophages [
24,
36].
Alveolar bone resorption (loss) is a notable radiographic indication of chronic AP. The alveolar bone functions to hold teeth in the socket while absorbing any force applied to the teeth during biting and chewing. Subject to varying degrees of mechanical forces, the alveolar bone undergoes repeating cycles of bone formation and resorption. In a state of proinflammation, this equilibrium tends towards bone resorption, resulting in the loss of both mineral and organic components of the alveolar bone. The close association of macrophages with different stages of AP implicates these cells in the development of alveolar bone resorption. Macrophages were observed near osteoblasts in rat models of AP during the initial stages of bone resorption, while no macrophages were seen near the surfaces undergoing bone formation [
19].
Osteoclastogenesis, or the development of mature osteoclasts (bone resorptive cells) from precursor cells, is mediated by receptor activator of nuclear factor kappa-β ligand (RANKL) [
38]. RANKL is a cytokine produced by osteoblasts (which synthesize bone matrix and differentiate into osteocytes [
39]) and stromal cells that binds to its receptor, RANK, to initiate the differentiation of osteoclast precursor cells [
40]. This process is regulated by Osteoprotegerin (OPG), a decoy receptor of RANKL that is primarily produced by osteoblasts [
41]. Hence, the RANKL-RANK-OPG trio are the main players in bone modeling and remodeling pathways [
42]. GM-CSF (Granulocyte-Macrophage Colony-Stimulating Factor) is a cytokine that induces proliferation and maturation in macrophages, and it has been found to promote fusion of prefusion osteoclasts in the presence of RANKL, enabling bone resorption under pathologic and inflammatory conditions [
43,
44].
4. Sensory Neuronal Activation in Apical Periodontitis
Periradicular tissues have an abundant supply of neurons that originate in the trigeminal ganglion (TG) [
52]. Research groups in the 1970s identified nociceptive afferents with different activation thresholds and conduction velocities in the PDL [
53]. Immunocytochemical analysis identified NGF-positive sensory nerves in periodontal tissues from rats [
54], while ultrastructural analyses of the human PDL using electron microscopy identified Ruffini nerve endings, free nerve endings, and lamellated corpuscles [
55]. Electrophysiological studies performed on cat models helped characterize trigeminal nerve endings in the PDL into low-threshold mechanoreceptors sensitive to mechanical load and vibration (A-β fibers) and nociceptors sensitive to pressure, temperature, and chemical irritants (A-δ, C-fibers) [
56,
57]. A-β fibers have large cell bodies, and these are myelinated [
58,
59]. A-δ fibers have medium-sized cell bodies, and C-fibers are considered the smallest [
58,
59,
60]. Myelinated A-δ fibers have higher conduction velocities, resulting in a sharp pain sensation compared to the unmyelinated, slow C-fibers, which relay a delayed and dull, ache-like pain [
58,
61].
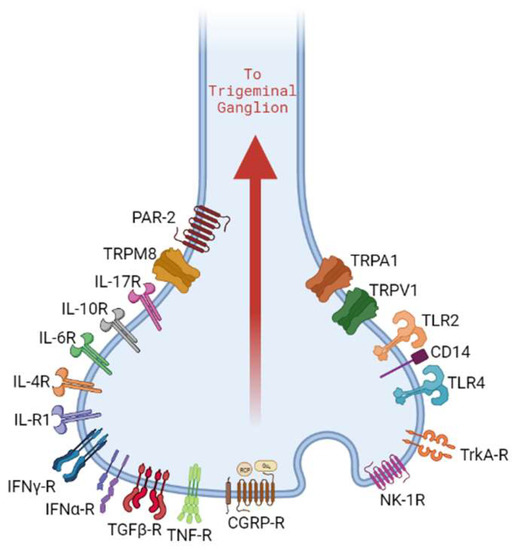
Specialized surface receptors expressed by nociceptors play a crucial role in the cascade of events that led to NI (
Figure 1). Transient receptor potential (TRP) channels are a class of integral membrane proteins that function as signal transducers in sensory neurons. These are polymodal in nature and can be activated by intracellular and/or extracellular mechanical, thermal, chemical, and osmotic pressure gradients. Based on sequence similarity, several classes of TRP channels exist. The TRPV subfamily (vanilloid subtype) has six members, of which TRPV1 has garnered attention for its role in the detection of noxious physical and chemical stimuli [
67,
68]. TRPA1 is the sole member of the TRPA subfamily (ankyrin subtype) [
69,
70]. The detailed 3D structure of the TRPA1 receptor was previously characterized by Cao et al. and Liao et al. [
71,
72].
Figure 1. Nociceptor neurons express receptor molecules that recognize PAMPs, DAMPs, cytokines, and neuropeptides. This results in the activation of downstream signaling pathways that can contribute to neurogenic inflammation.
Toll-like receptors (TLRs) are another class of surface receptors that have been identified on nociceptors. The nociceptor expression of TLR4 highlights its role in the host immune response to bacterial infections [
81,
82]. Peripheral sensory neurons showed the colocalized expression of TLR4 and CD14, indicating the capability of the direct detection of PAMPs [
82]. Besides immunological implications, the LPS binding of TLR4 on trigeminal neurons was shown to increase sensitization of TRPV1 channels, leading to increased expression of CGRP, which suggests a possible mechanism for pain associated with bacterial infections [
83,
84,
85].
5. Role of Sensory Neuropeptides in Pain and Inflammation
CGRP was discovered in 1982 by Amara et al. [135]. This 37-residue peptide was found to be expressed in the central and peripheral nervous systems, promoting sensory, motor, and autonomic functions in the brain [136]. CGRP has two isoforms, α-CGRP and β-CGRP, which have over 90% similar homology and share similar biological effects in humans [75]. CALC I gene undergoes alternate splicing to generate α-CGRP, while β-CGRP is a product of CALC II gene transcription. While α-CGRP is predominant in the central and peripheral nervous systems, β-CGRP is found mainly in the enteric nervous system [75].
The CGRP receptor molecule is a G protein-coupled receptor and is composed of three components: receptor activity modifying protein 1 (RAMP1), Calcitonin-like receptor (CLR), and receptor component protein (RCP). All three components are required to form a fully functional CGRP receptor (CGRP-R). Upon activation by binding with CGRP, CGRP-R may couple with G protein α subunit, increasing the cyclical adenosine monophosphate (cAMP) levels, which activate protein kinase A (PKA).
Substance P (SP) is an undecapeptide that was first discovered in 1931 by Von Euler and Gaddum [140]. SP, neurokinin A (NKA), neuropeptide K (NPK), and neuropeptide γ (NPγ) are tachykinin neuropeptides encoded by the TAC1 gene; alternate RNA splicing and differential post-translational processing result in the expression of specific tachykinin peptides [141]. SP is best known for its role as a modulator of pain perception by altering cellular signaling pathways using G protein-coupled receptors called neurokinin 1 receptor (NK-1R) that can act through the cAMP secondary messenger system or inositol triphosphate (IP3)-diacylglycerol (DAG) system [108].
CGRP and SP are known to colocalize, and these have been studied to assess the degree of pain experienced by patients with symptomatic AP [
142]. Affirming their role in oral diseases, several studies have reported increased levels of CGRP and SP in the sites of inflammation in the dental pulp [
143,
144], periodontal ligament [
145,
146], and saliva and gingival crevicular fluid [
111,
147,
148]. The levels of neuropeptides in these environments are correlated with the degrees of inflammation, confirming their role in inflammatory mechanisms [
111,
113,
144].
CGRP and SP play vital roles in modulating bone resorption. In murine AP models, TRPV1-positive sensory neurons were shown to regulate osteoclastogenesis via CGRP signaling [
104].
SP and CGRP have also been shown to mediate tissue repair. The sprouting of CGRP-positive fibers in odontoblast layers after injury induced in the cervical dentin of rats resulted in the formation of reparative dentin [
149]. SP released by stimulated peripheral nerves increased blood flow to the dental pulp, thereby enabling tissue healing [
113,
116]. Hence, SP and CGRP can be considered immunomodulators that can have pro- and anti-inflammatory effects depending on molecular cues present in the tissue microenvironment.
6. Neuro-Immune Interactions Modulate Inflammation and Healing in AP
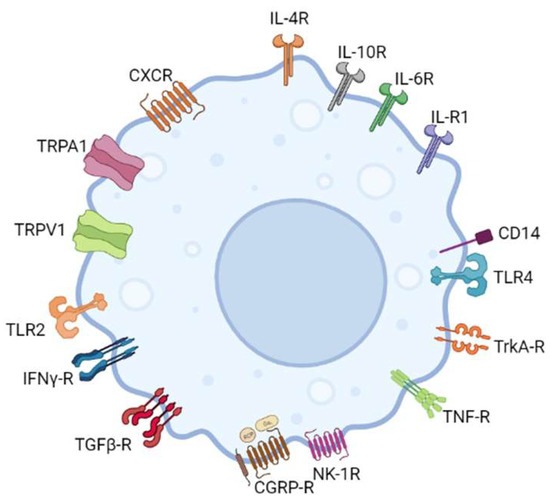
Evidence of a functional link between sensory neurons and immune cells comes from the proximity of sensory nerve endings and immune cells. This is corroborated by the expression of neuropeptide-specific receptors on immune cells and cytokine receptors and receptors capable of recognizing microbial pathogens on trigeminal nociceptor neurons [
8,
150] (
Figure 1 and
Figure 2).
Figure 2. In addition to releasing neuropeptides, macrophages have been shown to express neuropeptide-specific receptors, indicating a significant role in neuro-immune cross-talk.
In vitro studies have confirmed the expression of CGRP-R and NK-1R in murine and human macrophages, implying that neuropeptides CGRP and SP influence the activities of these immune cells [
99,
151]. Pro-inflammatory cytokines IL-1, IL-4, and interferon (IFN)-γ were also shown to induce NK-1R expression in macrophages [
152,
153]. Goldman et al. were among the first to conduct in vitro studies on peritoneal macrophages, showing enhanced phagocytosis when exogenous SP was applied to these cells [
154]. The SP-mediated activation of macrophages was discovered when exposure to SP increased levels of eicosanoids in macrophages [
155]. SP also acts as a chemoattractant for monocytes and macrophages, first demonstrated in vitro using human monocytes and peritoneal macrophages from guinea pigs [
156,
157]. Evidence of TNF-α, IL-1, and IL-6 release in macrophages in response to SP was also previously recorded [
158].
Early identification of CGRP immunoreactive (CGRP-IR) nerve fibers in the PDL led to increased interest in studying their response to different stimuli. Dynamic changes were observed in CGRP-IR nerve densities surrounding blood vessels in PDL during tooth movement in rat molars [
159]. Wakisaka et al. were among the first to study the distribution of SP-positive nerve fibers in rat PDL [
160]. However, when immunoreactions for CGRP, SP, and neuropeptide Y (NPY) were evaluated in dental pulp, periodontal ligament, and gingiva in cats, denser innervation in the pulp was reported, and these nerves were found to be more immunoreactive to CGRP than to SP [
161].
Dynamic changes in the distribution of sensory nerves during acute and chronic AP have drawn attention to understanding how these specialized neurons may modulate disease progression. Several studies measured increased levels of neuropeptides in saliva and gingival crevicular fluid (GCF) in animal models and in patients diagnosed with AP [
165,
166]. Similarly, investigations on the distribution patterns of neuropeptides in periodontitis suggested the potential of using neuropeptides as biomarkers for the early detection of periodontitis [
167,
168].
Macrophages express functional CGRP-R, i.e., receptors of CGRP. CGRP activation led to the proliferation and differentiation of murine macrophages into osteoclast-like cells via a cAMP/PKA-dependent signaling pathway [
172]. Murine bone-marrow-derived macrophages (BMDMs) showed increased IL-6 expression as a response to CGRP activation, which led to speculations about the role of BMDMs in the negative inhibition of B cell development [
173]. Similar findings were reported in rat models that demonstrated reduced IL-6 expression in invading macrophages upon perineural injection of a CGRP antagonist [
100].
Primarily produced by sensory neurons in the periodontal tissues, NGF is a neurotrophic factor that plays a critical role in neurogenesis, promoting the growth, differentiation, and survival of neurons [
174,
175]. Nerve Growth Factor-Tropomyosin receptor kinase A (NGF/TrkA) signaling suppresses CGRP production in monocytes as well as RAW264.7 macrophages [
99]. In periodontal tissues that have a rich sensory nerve supply, NGF can induce increased neural sprouting and innervation under inflammation. NGF also controls the expression and release of neuropeptides in sensory neurons. NGF-mediated neural responses have direct implications on the host tissue response to inflammation [
176,
177]. NGF has been shown to induce monocyte differentiation in macrophages, thereby enhancing inflammatory effects [
178].
SP was found to bind NK-1R receptors on murine macrophages and activate protein kinase C (PKC) or phosphatidylinositol 3-kinase/protein kinase B (PI3K/Akt), which triggers nuclear factor kappa-light-chain-enhancer of activated B cells transactivation and chemokine response via ERK1/2 and p38 MAPK signaling pathways [
185,
186]. Both resulted in the expression of the chemokines macrophage inflammatory protein-2 (MIP-2) and MCP-1. MIP-2 is a potent chemoattractant for neutrophils, and MCP-1 regulates monocyte migration and infiltration [
187,
188]. Macrophages produce SP in response to NF-κB activation, linking this phenomenon with the pro-inflammatory M1 macrophage phenotype. The administration of exogenous SP has been found to increase IL-10 expression in rat models, creating conducive environment for the activation of anti-inflammatory M2 macrophages, which can also produce SP [
189,
190].
7. Association of Pain with Nociceptor-Macrophage Cross-talk in AP
Pain is a natural response to tissue damage, and current investigations highlight a close association of this sensation with nociceptor–immune-cell interactions [
194]. Several aspects can contribute to the presentation of pain in endodontic infections, including the volume of root canal space, the presence of pathogen diversity, the virulence of pathogenic load, the state of the immune system in an individual, and lesion dynamics, among others [
195]. Despite standardized systems such as Numeric Rating Scale (NRS-11) and periapical index (PAI), which can correlate pain with clinical presentations in AP, these cannot be used to predict when and why pain is not experienced by patients with chronic AP [
196,
197].
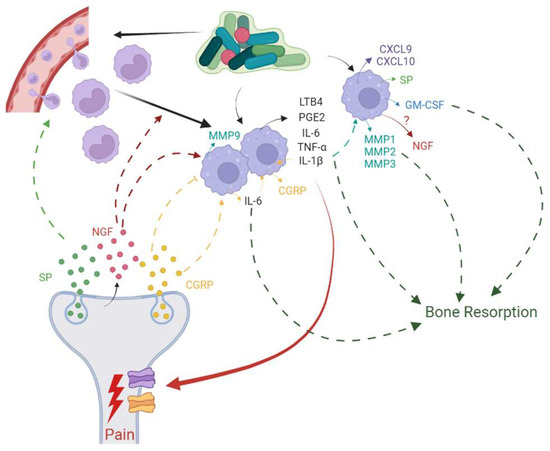
Immune cells modulate nociceptor activity using cytokines and chemokines, and nociceptors in turn modulate functions of immune cells by expressing neuropeptides, resulting in a dynamic, bi-directional cross-talk between these two cell entities during tissue injury and inflammation [
198] (
Figure 3). Nociceptors express receptor molecules that are activated by PAMPs, DAMPs, and mediators released by immune cells. Neutrophils, mast cells, and macrophages have close encounters with nociceptive nerve terminals, and hence, mediators produced by these immune cells can induce pain sensitization and influence chronicity of pain. Macrophages produce TNF-α, IL-1β, IL-6, PGE2, and Leukotriene B4 (LTB4), covering a range of cytokines, growth factors, and lipids that bind to specific receptors on nociceptor neurons and result in direct pain sensitization or engage ion channels to increase neuronal cell excitation which then causes pain sensitization [
194]. Such pain sensitization translates to hyperalgesia or allodynia in AP.
Figure 3. Bi-directional cross-talk between nociceptor neurons and macrophages has been implicated in inflammation, resulting in pain and bone resorption in apical periodontitis.
This entry is adapted from the peer-reviewed paper 10.3390/biom13081193