Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Food Science & Technology
Aspartame is the methyl-ester of the aspartate-phenylalanine dipeptide. Over time, it has become a very popular artificial sweetener. However, since its approval by the main food safety agencies, several concerns have been raised related to neuropsychiatric effects and neurotoxicity due to its ability to activate glutamate receptors, as well as carcinogenic risks due to the increased production of reactive oxygen species.
- aspartame
- artificial sweetener
- excitotoxicity
- neuropsychiatric symptoms
- reactive oxygen species
- carcinogenic risk
1. Introduction
Due to decreased sugar production throughout the two world wars as well as an increased prevalence of nutrition disorders, particularly diabetes mellitus, in post-war industrialized societies, artificial sweeteners, also known as non-nutritive sweeteners, gained popularity [1]. Some non-nutritive, low-calorie sweeteners provide a similar taste while bringing 200–300 times fewer calories than sugar [2]. Saccharin, discovered and used since 1879, was widely regarded at the time as a sugar substitute. James Schlatter, while doing biochemical synthesis experiments with Robert Mazur, accidentally discovered aspartame in 1965, and his employer G.D. Searle immediately started testing the substance in the hope of producing and commercializing it on a wide scale [3,4] (Figure 1). Aspartame is the methyl ester of the dipeptide formed by L-aspartic acid and L-phenylalanine [5]. It has been found to be 188 times sweeter than sugar while having the same calorie contents per weight unit [6].
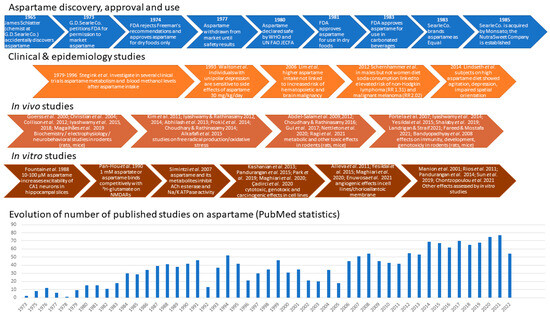
Figure 1. Main steps related to aspartame discovery, approval, development, and use, along with some of the clinical trials, in vivo and in vitro studies evidencing its biological effects, and the dynamics of publications describing them [7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59].
In 1973, D. Searle requested from the Food and Drug Administration (FDA) an initial clearance for aspartame. The FDA stated that the maximum daily dosage of aspartame for humans should be 50 mg/kg body weight/day in the United States; in Europe, a maximal acceptable daily intake (ADI) of 40 mg/kg body weight/day was adopted [60]. However, some chronic exposure and carcinogenesis studies [61,62] found—even in female rats exposed to relatively low doses of aspartame (4 or 20 mg/kg body weight/day)—exposures that are close to the current ADI in the European Union (40 mg/kg body weight/day) bring an increased incidence of malignant tumors.
By 1983, the Equal® brand of aspartame sugar replacement ruled the tabletop non-nutritive sweetener industry. Aspartame appealed to diabetics because its dipeptide composition did not require insulin to be metabolized, and it featured a better sweet taste devoid of bitterness and presumably fewer side effects compared to earlier sugar substitutes, such as saccharin and cyclamate. Large soft drinks companies, such as Coca-Cola® and Royal Crown®, declared they would combine two artificial sweeteners, e.g., aspartame and acesulfame K, in their low-calorie diet soda products in order to minimize the side effects of each of them [1,7,63], a guideline followed so far. One year after full approval was obtained, in 1984, NutraSweet® quickly developed into a very lucrative division of G.D. Searle, and the number of customers grew less affected by safety concerns [64]. Thus, nowadays, aspartame is a common component in over 6000 food products and beverages.
Aspartame is present in soft drinks, dessert mixes, yogurt, chewable multivitamins, and morning cereals. Millions of people throughout the world ingest it because it is also present in 600 different types of medicines [65]. The production of low-calorie beverages, which are widely consumed by youngsters and pregnant people, is a crucial use in the United States [66,67]. Although some early studies estimated that the amount of methanol intake resulting from diet soda drinking in a hot environment could reach 250 mg/day or 32 times the Environmental Protection Agency’s daily limit suggestion [68], a more realistic estimate places the methanol intake resulting from daily aspartame consumption in the highest 90% as being 25 times lower than the maximal safe level of methanol intake of 7.1–8.4 mg/kg/day established by FDA, and much lower than the methanol intake resulting from other natural sources, such as pectin, fruits, vegetables, and alcoholic beverages [69]. A substantial body of the literature shows that young animals are more vulnerable than older animals to a variety of chemical and physical carcinogens, particularly during the prenatal period [70]. A re-examination of histopathology data from large groups of animals fed on aspartame-containing diets within studies performed at the Ramazzini Institute in Bologna confirmed that aspartame exposure during pregnancy raises the risk of cancer in rodent offspring [8]. Additional research into associations between aspartame and conditions, like brain tumors, brain lesions, and lymphoma, has also been advised by several researchers [61,71]. The Food Additives and Nutrient Sources Added to Food Panel evaluated the potential risks of aspartame for pregnant women by assessing the plasma concentrations of the breakdown product phenylalanine following the consumption of aspartame-containing products [60,72] (Figure 2).
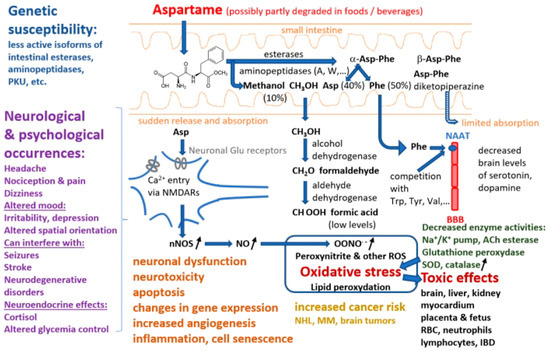
Figure 2. Summary of pathways involved in aspartame decomposition, kinetics, metabolism, potential adverse effects at cell level, side effects, and relationships with different disorders (abbreviations: PKU—phenylketonuria, NAAT—large neutral amino acids transporter, BBB—blood–brain barrier, NMDARs—N-methyl-D-aspartate receptors, nNOS—neuronal nitric oxide synthase, NO—nitric oxide, ROS—reactive oxygen species, ACh—acetylcholine, SOD—superoxide dismutase, RBC—red blood cell, IBD—inflammatory bowel diseases, NHL—non-Hodgkin lymphoma, MM—multiple myeloma).
2. Chemical Structure, Digestion, and Metabolism
Aspartame is the methyl ester of a dipeptide composed of a hydrophilic and a hydrophobic amino acid residue, aspartic acid (Asp) and phenylalanine (Phe), respectively, giving it some unique qualities [73,74]. Aspartame in purified solid form is a white crystalline powder that may be stored at temperatures between 30 and 80 °C and is extremely stable under dry conditions [75]. At room temperature, its aqueous solution has a half-life of approximately 300 days and reaches the highest stability at a pH of 4.3, which is common for diet sodas. The peptide bonds are hydrolyzed in certain conditions, such as high temperature or basic pH [1].
Aspartame stability in soft drinks has been studied intensively. Thus, it was found that after 50 weeks storage at room temperature of a diet soda, 20% of its aspartame content was de-esterified to α-Asp-Phe, 15% was converted to β-Asp-Phe and β-aspartame, and another 20% was converted into a cyclic dipeptide known as aspartame diketopiperazine (3-carboxyl-methyl-6 benzyl-2.5 diketo-piperazine) [75,76]. Aspartame produces methanol by hydrolysis in highly acidic or alkaline environments. The peptide bonds are also hydrolyzed in more extreme circumstances, releasing the free amino acids. Upon consumption, aspartame is split by several digestive enzymes, such as esterases and peptidases, into a number of chemical components, including aspartic acid, phenylalanine, and methanol, the latter being further decomposed into formaldehyde and formic acid [77]. Studies with human and pig intestinal microvilli preparations and specific inhibitors showed that aminopeptidases A and W are the most active in decomposing the α-Asp-Phe dipeptide [78]. Another pioneering study proved that Asp-Phe is hydrolyzed by three of four brush border peptidases and by a cytosolic peptidase different from the seven known isoforms [5]. Similarly, amino acids and dipeptide intestinal absorption studies [79] showed that, although dipeptide absorption mechanisms are present, particularly in the jejunum and ileum [80], α-aspartame is almost entirely decomposed in the intestinal lumen and passes into circulation as Asp (40%), Phe (50%), and methanol (10%) [69,76]. The rates of intestinal absorption of β-Asp-Phe and aspartame diketopiperazine are small [76]. The group of Lewis Stegink was particularly active and performed a number of clinical studies on adults, children, and infants, involving acute (single-dose or repeated doses over less than 1 day) or prolonged (e.g., daily for 13 consecutive weeks) intake of aspartame doses, sometimes higher than the ADI (up to 200 mg/kg body weight) to assess the pharmacokinetics and demonstrate the lack of toxicity of aspartame decomposition products [9,10,11,12,13,14,15,16,81], except for subjects with genetic traits resulting in low plasma α-Asp-Phe hydrolase activity [17]. However, other clinical studies reached different conclusions, showing adverse effects of aspartame, particularly in subjects with neurological or psychiatric conditions, such as migraines [82,83], other headaches [84,85], or unipolar depression [18]. Part of these differences may result from the fact that aspartame was administered in some studies, e.g., [18], as powder included in enteric-soluble capsules, which can release very high concentrations of aspartame over limited areas of the intestinal mucosa, in contrast to administration in a pre-dissolved form in water or beverages.
While phenylalanine is turned into tyrosine and phenylethylamine, and methanol is converted into formaldehyde, which then undergoes an oxidation reaction to formic acid, aspartic acid is converted to alanine and oxaloacetate [86]. Each of these compounds is metabolized according to a natural metabolic route in the same manner as those originating from foods and other dietary sources. As demonstrated in animals, methanol from aspartame enters the portal circulation and is promptly converted by alcohol dehydrogenase to formaldehyde, which is further transformed into formate by aldehyde dehydrogenase [65]. Early pharmacokinetics and metabolism studies in humans have shown that upon acute ingestion of 50 mg/kg aspartame, blood methanol levels increased to 0.34 ± 0.12 mg/dL (mean ± SEM, n = 6) in adults 30–90 min after intake, and to 0.30 ± 0.10 mg/dL in infants; higher aspartame doses produced proportionally higher peak blood methanol levels [9,10]. However, several researchers have pointed out that methanol levels resulting from aspartame intake are several times smaller than those produced by consumption of other common foods and drinks, like fruit or vegetable juices and fermented distilled beverages, due to enzyme-driven breakdown of methoxyl groups of polysaccharides, such as pectin [9,69]. Therefore, aspartame side effects are more likely due to the two amino acids released by its decomposition, phenylalanine, and aspartate. Thus, a clinical study on children fed with aspartame 34 mg/kg/day for two weeks proved increased phenyalanine and tyrosine plasma levels compared to a placebo group [87].
The increased phenylalanine concentrations are linked to lower levels of catecholamines, serotonin, and dopamine [88]. Phenylalanine is a large neutral amino acid that competes with other amino acids for binding on the large neutral amino acid transporter [89]. Phenylalanine released from aspartame may theoretically mediate neurologic effects since it has neurotoxic potential and influences the production of monoamine neurotransmitters. When pentylenetetrazole, an epileptogenic drug, is administered to mice after aspartame administration, the frequency of seizures that follow is increased [90]. This is because aspartame causes plasma phenylalanine levels to rise more than those of tyrosine (which likely happens after any aspartame dose in humans). Phenylalanine prevents dopamine release in the striatum, whereas valine, which competes with phenylalanine for passage across the blood–brain barrier, can alleviate its proepileptogenic effect [91]. The reduced levels of dopamine and serotonin are a result of the excess phenylalanine blocking the transport of crucial amino acids to the brain. In addition to being employed in protein synthesis, phenylalanine can also be converted into the highly concentrated phenylpyruvic acid in phenylketonuria patients [19,92]. By competing for neutral amino acid transporters, phenylalanine can directly affect the entry of other critical amino acids into the CNS. As a result, it indirectly influences neurotransmitter deficiencies that result in functional problems [93].
On the other hand, aspartate, the carboxylate anion of aspartic acid, undergoes transamination in enterocytes to become oxaloacetate before reaching the portal circulation [69]. The urea cycle and gluconeogenesis can be affected by the body’s conversion of oxaloacetate and aspartate [2]. Aspartate and other related amino acids, such as asparagine, glutamate, and glutamine, did not significantly change their plasma levels in healthy people after taking aspartame doses of 34–50 mg/kg [14]. Aspartic acid residues are frequently found in proteins. The body may convert aspartic acid into the neurotransmitter glutamate, which at very high levels, can have harmful effects on the nervous system. In addition, high doses of aspartate can directly activate N-methyl-D-aspartate (NMDA) receptors, exerting excitotoxicity and other central nervous system adverse effects. However, the European Food Safety Authority’s experts did not see any evidence of neurotoxicity associated with aspartame and therefore concluded that aspartic acid derived from aspartame does not raise any safety concerns for consumers [60].
3. Mechanisms of Toxicity of Aspartame Metabolism Products
The fundamental tenet of toxicology is that all substances are harmful at some concentration. As a result, it is not surprising that aspartame or its components have negative effects on sensitive animal species when consumed at very high doses. Upon testing the effects of various aspartame doses on blood levels of aspartate, phenylalanine, and methanol, several studies proved that these levels were well below those associated with adverse effects in animal species, raising the important question of whether aspartame ingestion is potentially harmful to humans during normal use or abuse, in spite of the fact that the dietary exposure of consumers to these compounds is higher than that resulting from aspartame intake [12]. Although FDA and other regulatory agencies have established permissible daily intake guidelines for aspartame ingestion, there are many questions about its safety today.
Frequent high-dose aspartame intake may have nephrotoxic effects. Thus, according to experimental data from different animal species, long-term consumption of aspartame caused a dose-dependent increased production of free radicals in renal tissues as well as kidney injury, as proved by a search of several literature databases for publications on the adverse effects of aspartame on the kidney function from 1980 to 2016 [94]. Additionally, recent cohort studies showed a link between excessive aspartame use and an elevated risk for cardiovascular disorders [95]. The administration of aspartame caused oxidative stress and markedly reduced the activity of antioxidant enzymes, such as superoxide dismutase, catalase, glutathione peroxidase, and glutathione reductase in both rat liver and renal tissues [96]. Increased pro-oxidant levels, such as reactive oxygen and nitrogen species (ROS/RNS), or decreased antioxidant levels, which could cause cell malfunction and disintegration, are indicators of oxidative stress [97].
4. Neurological and Cytotoxic Effects by Activation of NMDA and Other Glutamate Receptors by Aspartame or Its Metabolites
Glutamate represents the main excitatory neurotransmitter in the central nervous system. Glutamate receptors are divided into the following two groups: metabotropic glutamate receptors (mGluRs), with seven transmembrane α-helical segments accommodating the ligand molecule at the center, similar to rhodopsin, and ionotropic glutamate receptors (iGluRs), tetrameric ligand-gated ion channels with large extracellular domains featuring multiple ligand-binding sites and four transmembrane α-helical segments per subunit. The three types of ionotropic glutamate receptors—NMDA, AMPA, and kainate receptors—are distinguished by varying ion selectivity, activating agents, and pharmacological agonists and inhibitors [109]. The N-methyl-D-aspartate receptors (NMDAR) are crucial molecular components of learning and memory via the complex phenomenon of long-term potentiation (LTP), which involves receptor phosphorylation triggered by calcium influx upon repeated stimulation [110]. However, several pathological conditions, such as ischemic stroke or neurodegenerative diseases, may lead to excitotoxicity, consisting of excessive synaptic glutamate release and NMDAR overactivation with massive Ca2+ inflow, resulting in neuronal cell death [111]. The same author pointed out that the developing human brain is exposed to excitotoxic compounds, such as those present in foods, to a much larger extent compared to the adult brain due to an immature blood–brain barrier [112]. Therefore, it seemed logical to express similar concerns over the use of aspartame since the compound itself and its decomposition product aspartate may effectively activate NMDARs [113], in addition to neurotransmitter imbalances caused by aspartate and phenylalanine [88,91].
This entry is adapted from the peer-reviewed paper 10.3390/nu15163627
This entry is offline, you can click here to edit this entry!
