Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Polymer Science
Polyurethane elastomer (PUE) is a typical block polymer with alternately arranges soft and hard segments. Isocyanates and small molecular chain extenders constitute the hard segment, and the soft segment is composed of polyols. Most phosphorous flame retardants for PUE exhibit two flame-retardant mechanisms, including the gas phase and the condensed phase.
- polyurethane elastomer
- phosphorus flame retardant
- reactive flame retardant
1. Introduction to Polyurethane Elastomers
Polyurethane elastomer (PUE) is a typical block polymer with alternately arranges soft and hard segments [1,2]. Isocyanates and small molecular chain extenders constitute the hard segment, and the soft segment is composed of polyols. Compounds containing two or more -OH groups with a molecular weight of more than 500 g/mol are defined as polyols, while those smaller molecules with hydroxyl groups and/or amines are regarded as chain extenders.
The abundant hydrogen bonds physically cross-link the linear chains to form network structures, resulting in microscopic phase separation between soft and hard segments [3]. The mobility of the soft segment makes the PUE elastic, while the hard segment hinders the rotation of the molecular chain, giving the PUE hardness and mechanical strength [4]. The unique micro-phase structure of PUE endows it with wear resistance, toughness, and good processability of thermoplastics [5]. The combination of “tough and strong” greatly expands the application of PUE.
The soft segment offers the flexibility of PUE chains. The polyols used to synthesize PUE can be classified into polyether polyols, polyester polyols, and polyolefin polyols. Polyester polyols can be further divided into aliphatic polyester polyols and aromatic polyester polyols [7]. Polyolefin polyols are polyols containing -C=C- bonds in the repeating units, including polybutadiene polyols, polybutadiene acrylonitrile polyols, and the like. The comparisons of various types of polyols are summarized in the table below (Table 1).
Table 1. Classification and comparison of the advantages and disadvantages of various polyols (↑ represents increase and ↓ represents decrease) [8].
| Type | Representation | Advantage | Disadvantage |
|---|---|---|---|
| Polyether polyol |
PEG | Hydrolytic stability↑, cost↓, flexibility↑ | Oxidative stability↓, strength↓, flammability↑, thermal stability↓, oxidative stability↓, cost↑ |
| PPG | |||
| PTMEG | Hydrolytic stability↑, strength↑ | ||
| Polyester polyol |
Aliphatic polyester polyol | Oxidative stability↑, strength↑ | Hydrolytic stability↓ |
| Aromatic polyester polyol | Thermal stability↑, stiffness↑ | Flexibility↓ | |
| Polyolefin polyol |
Polybutadiene Polyol | Low-temperature flexibility↑, solvent resistance↑ |
Cost↑ |
The hard segment of polyurethane elastomer is usually composed of isocyanate and chain extender, and the additional reaction of alcohol (-OH) to isocyanate (-NCO) is the basis for synthesizing polyurethane. PUE is generally synthesized with di-functional isocyanates, shown in Table 2 [9,10]. Aromatic isocyanates, such as toluene di-isocyanate (TDI) and methylene diphenyl di-isocyanate (MDI), have high reactivity and low production costs, and they are usually used to manufacture rigid products. However, products made from aromatic isocyanates tend to turn yellow over time due to their photo-oxidative instability. In contrast, products based on aliphatic di-isocyanates have excellent hydrolysis resistance and yellowing resistance in spite of their low reactivity and comparatively high expense. The common aliphatic isocyanates include hexamethylene di-isocyanate (HDI), isophorone di-isocyanate (IPDI), and 4,4′-diisocyanate dicyclohexylmethane (HMDI), which is a hydrogenation product of MDI [8].
Table 2. Di-isocyanate classification, advantages, and disadvantages.
| Type | Representation | Advantage | Disadvantage |
|---|---|---|---|
| Aromatic di-isocyanate |
TDI | High reactivity, low production cost |
Photo-oxidative instability |
| MDI | |||
| Aliphatic di-isocyanate | HDI | High chemical stability, excellent weather resistance | High production cost, low reactivity |
For most polyurethanes (poly(urea-urethanes)), soft segments and isocyanates account for more than 95% of the mass, and they are linked by lower molecular weight diols or di-amines chain extenders. The most commonly used chain extenders include 1,4-butanediol (BDO), ethylene glycol, and so on.
There are many choices of soft segments and hard segments for synthetic polyurethane elastomers, so adjusting the type and proportion of starting materials offers polyurethane elastomer a wide range of properties, synthesis routes, and processing methods. Contemporarily, coating materials based on polyurethane elastomers are widely used in various fields, such as construction, transportation, electronics, and aerospace, because of their good wear resistance, oil resistance, high elasticity, and high transparency [8,11,12]. However, the thermo-stability of polyurethane elastomer is not as desirable, and it has been defined as a flammable polymer [13]. A large number of toxic fumes causing poisoning and suffocation, such as CO, HCN, NOx, and –NCO group-containing compounds, are produced in the combustion of PUE [14,15,16], which causes great harm to human health and property safety. In 2021, a total of 748,000 fires were reported in China, with more than 4000 casualties and direct property losses of CNY 6.75 billion. The frequent occurrence of fires drives the attention of various industries to the flame retardancy of PUE materials [17,18,19].
2. Flame Retarding Mechanism of P-FRs in PUE
2.1. Thermal Decomposition Behavior of P-FR Polyurethane
Once the polyurethane is ignited, toxic gases are produced with significant life hazards, so flame retardancy and thermal stability of PU are extremely important. Thermal decomposition of PU is divided into at least two stages [15,54]. Firstly, carbamate bonds are cleaved to transform polyurethanes into oligomers and small molecules, such as isocyanates, alcohols (amines), and carbon dioxide. When the temperature continues to rise, the soft segments (polyols) begin to decompose, while diffusing into the flame area, and form flammable blends with oxygen in the air to promote combustion.
Generally, with the addition of P-FRs, the thermal decomposition temperature and thermal stability decrease, with a few exceptions documented in recent reports [55,56]. Thus, whether the thermal stability of flame retardants matches that of the matrix is an important consideration. Alleviation or prevention of matrix degradation will only be effectively achieved if the flame retardant decomposes first.
2.2. General Flame Retarding Mechanisms of P-FRs
Most flame retardants exhibit two flame-retardant mechanisms, including the gas phase and the condensed phase (shown in Figure 4). Both of them effectively prevent the spread of flames and ensure the safety of people’s lives and properties.
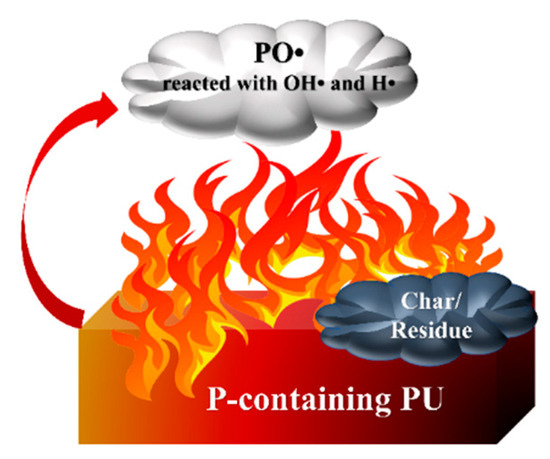
Figure 4. Gas and condensed phase flame-retardant mechanisms of P-FRs.
In the condensed phase, phosphorus-containing compounds are turned into phosphoric acid and poly-phosphoric acid upon heating. Phosphoric acid has a strong dehydration ability, which promotes carbonization to form a closed carbon layer. Meanwhile, water, the other product of this transformation, absorbs heat and vaporizes, reducing the temperature around the material.
Meanwhile, the phosphorus-containing polymer releases incombustible gas during the decomposition process to dilute the concentration of combustible gas, which is usually defined as the gas-phase flame-retarding route. When the polymer is burned, a large number of radicals, such as H• and OH• radicals, are generated, which triggers a series of chain reactions and aggravates the combustion. Many P-containing flame retardants form PO•, which reacts with OH• and H• radicals, reducing their concentration and interrupting the combustion reaction. Most phosphorus-containing flame retardants have synergistic flame retardancy in both the condensed and gas phases [22].
2.3. Effect of Phosphorus Oxidation State on Flame Retarding Mechanism
Phosphorus has different oxidation states (−1 to +5), which give rise to different flame-retardant mechanisms, as shown in Figure 5. When the substituting groups are basically the same (for example, R1 = R2 = R3), the phosphorus content (weight percent in the whole molecule) decreases while the oxidation state increases. Modesti et al. [57] investigated the effect of the P oxidation state on its FR behavior in polyurethane foams, finding that the amount of stable residue after burning increased, together, with less release of volatiles as the oxidation state of P increased. Therefore, the low oxidation state generally led to the gas-phase FR mechanism, while the high oxidation state facilitated the condensed phase FR mechanism [58,59]. These insights have significant impacts on the design of flame retardancy for polymer materials based on phosphorous.
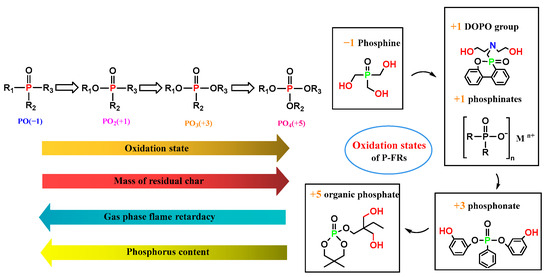
Figure 5. Different oxidation states impact flame-retardant behavior.
This entry is adapted from the peer-reviewed paper 10.3390/polym15183711
This entry is offline, you can click here to edit this entry!
