1. Innate and Adaptive Immune Cells in COVID-19 Patients
SARS-CoV-2 infection activates both innate and adaptive immune response, where severe inflammatory response may cause local tissue damage (acute lung injury) and ARDS at systemic level [
11,
12]. Therefore, the knowledge behind this enhanced activation of cytokine storm due to dysregulated immune function after SARS-COV-2 infection will provide ways to clinically manage and prevent its transmission from mild to severe stage. Notably, bronchial mucosal-associated invariant T (MAIT) cells and γδ T cells are the primary innate immune cells that can trigger cytokines response after SARS-COV-2 infection, especially in patients developing the severe disease [
13]. As a result of the activation of these innate immune cells and the consequential expression of pro-inflammatory cytokines genes, the host adaptive immune system becomes activated against virus infection.
Circulating white blood cells, including neutrophil, basophils, eosinophils numbers are consistently higher in survivors of COVID-19 than in non-survivors [
14,
15]. SARS-CoV-2 infection also induces lymphocytopenia in clinically severe patients that mostly affects the CD4+, CD8+ T cell subset, including effector, memory, regulatory T cells, and natural killer cells [
11,
12]. These observations are in line with the previous findings in severe or lethal cases of SARS-CoV and MERS [
16,
17]. This reduction in immune cells repertoire could be due to damage of lymphocytes or lymphocytic organs associated with infection with SARS-CoV-2 via their minimally expressed ACE2 receptors. Notably, the baseline levels of CD8+ T cells and NK cells are inversely correlated to ACE2 expression in human lung tissue [
18]. Cytotoxic T lymphocytes (CTLs) and natural killer (NK) cells are considered as a critical cell that controls viral infection. These cells were found to be exhausted in SARS-CoV-2-infected patients with a significant increase in the exhaustion marker PD1 (programmed death-1) compared to healthy controls and thus likely responsible for viral progression [
19,
20,
21]. A recent study from Yang et al. demonstrated clinical outcomes of 93 SARS-CoV-2-positive patients in association with neutrophil(NEU)-to-lymphocyte (LYM) ratio (NLR), lymphocyte-to-monocyte (MON) ratio, platelet-to-lymphocyte ratio (PLR), and C-reactive protein (CRP) expression. According to their study, the aged patient group with elevated NLR was significantly associated with illness severity and could represent an independent prognostic biomarker for COVID-19 patients [
22]. Similarly, another group in China with a cohort of 245 SARS-CoV-2-positive patients identified a patient group with elevated NLR, which was at a hyper-risk side compared to the other groups [
23].
2. High-Throughput Sequencing Approach to Understand Immune Cell Dysfunctions in SARS-CoV-2-Infected Patients
Recently, Wen et al. have comprehensively characterized changes in transcriptional landscape during the recovery stage of SARS-CoV-2 infection by single-cell RNA sequencing (scRNA-seq) using peripheral blood mononuclear cells (PBMC) [
24]. According to their study, inflammatory cytokines gene expressing CD14+ monocytes and plasma B-cell numbers were remarkably increased in COVID-19 patients during the early recovery stage [
24]. Mingfeng et al. characterized bronchoalveolar lavage fluid (BALF)-immune cells through scRNA-seq from patients with varying severity of SARS-CoV-2 (mild and severe). BALF from critical/acute SARS-CoV-2-infected patients showed a higher proportion of macrophages and neutrophils and a lower proportion of myeloid/plasmacytoid dendritic cells and T cells compared with moderately infected patients [
25]. Another study using scRNA-seq data identified specific cell types expressing a receptor for coronavirus infection (ACE2) across 13 tissue types; these include lung alveolar cells type-2, liver cholangiocyte, colon colonocytes, esophagus keratinocytes, stomach epithelial cells, and kidney proximal tubules [
26]. Importantly, it has been observed that disease conditions, such as chronic heart disease or chronic cigarette smoke exposure, showed enhanced ACE2 expression notified by scRNA-seq data in cardiomyocytes or human lung cells, respectively [
27,
28]. Notably, human testicular cells (spermatogonia, Leydig, and Sertoli cells) also predominantly express the ACE2 receptor [
29] representing that these tissue-specific cells are vulnerable to SARS-COV-2 infection [
30]. It is important to note that not only over- or under-expression of ACE2 receptor in human tissue could determine the susceptibility of patient cells to SARS-COV-2, but also ACE2 polymorphism could influence both the predisposition to infection and clinical outcome of the COVID-19 pathogenesis [
31]. Using mass-spectrometry, one study has profiled cellular immune components from SARS-CoV-2-infected patients with differences in disease progression (mild, severe, and critical) and compared with peripheral blood cells collected from healthy donors. According to their study, CD8+ T cells, dendritic cells, and macrophages were excessively activated initially during mild disease. They became exhausted in later critical stages, thereby representing disturbed homeostasis of the immune system during disease symptoms progression [
32].
The scRNA-seq approach has also been utilized to identify novel therapeutic regimens for SARS-CoV-2 infection. In this line, a bioinformatics pipeline has been developed, which integrates the scRNA-seq dataset with a drug perturbation database to identify potential therapeutic candidates for SARS-CoV-2 infection treatment. Using bioinformatics pipeline, four drugs—(1) didanosine, an HIV anti-viral drug; (2) benzyl-quinazoline-4-yl-amine, an EGFR inhibitor; (3) camptothecin, a topoisomerase inhibitor; and (4) RO-90-7501, an amyloid-β42 aggregation inhibitor—have been proposed as potential candidates to treat COVID-19 [
33]. Using knowledge from scRNA-seq and B-cell VDJ sequencing data, Cao et al. developed a therapeutic SARS-COV-2 neutralizing antibody (BD-368-2), with strong therapeutic and prophylactic efficacy in SARS-COV-2, and infected hACE2-transgenic mice [
34]. Further, using cryoelectron microscopy, the structure of BD-368-2 in complex with spike-ectodomain trimer was characterized, which revealed BD-368-2 binding to the ACE2 receptor [
34].
3. Hyper-Cytokine Activation
SARS-CoV-2 infection drives a profound cytokine response in the host, comprising a series of mediators that are targeted in immune-mediated inflammatory diseases (IMIDs) (
Figure 1). In some patients, a condition of hypercytokinemia also called a cytokine storm with SARS-COV-2 infection develops, which resembles secondary hemophagocytic lymphohistiocytosis, a hyper-inflammatory state triggered by viral infections [
35]. Recent reports have shown the level of plasma concentration of pro-inflammatory cytokines, such as TNF-α, IL-1β, IL-6, IL-8, IL-9, IL-10, bFGF, G-CSF, and GM-CSF, as well as chemokines, such as MCP1, IP10, and MIP1α, are elevated in patients with SARS-CoV-2 infection who are either admitted to an intensive care unit (ICU) or non-ICU patients compared to blood from healthy donors. These elevated cytokines levels were associated with lung injury [
11,
36]. Inflammatory cytokines were significantly elevated in patients who displayed severe clinical conditions and dismal clinical outcomes compared to moderately or mildly infected patients, thus suggesting that extensive changes in cytokines play a pivotal role in COVID-19 pathogenesis [
15,
37].
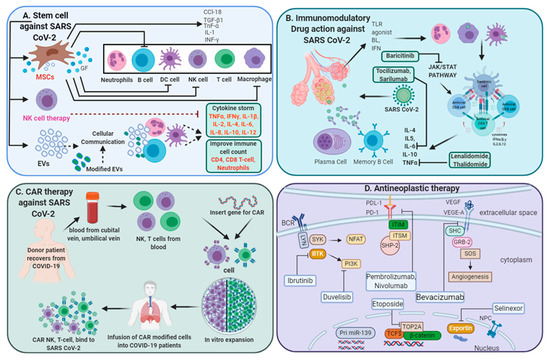
Figure 1. Multi-directive therapeutic intervention currently in clinical trials for combating SARS-CoV-2 pathogenesis. (A) Mesenchymal stem cell (MSC) and NK cell-based therapies for the treatment of SARS CoV-2 pathogenesis. Extracellular vesicles (EVs) derived from MSCs or modified exosomes containing antiviral/anti-inflammatory drugs or respective nucleic acid for therapeutic intervention to target cells. These cell-based or cell-derived therapeutic agents could modulate immune cell functions against SARS-CoV-2 infection. (B) Cytokine storm development is a central pandemic delinquent in SARS-CoV-2 infection. Certain immunomodulatory agents with excellent safety profiles or current anti-neoplastic interventions (D) may be considered for use in combination with antiviral drugs for the treatment of severe or critical COVID-19 cases. (C) Importantly, engineered immune cell receptors, including the chimeric antigen receptor (CAR) T-cell, NK cell therapy, offer new therapeutic approaches for SARS-CoV-2 infection. These immune cells collected from recovered patients offer an advantage as the majority of these cells have been primed with a viral antigen before collection and therefore transmit high proliferative efficiency and anti-viral efficacy.
4. SARS-COV-2 Severity in Cancer Patients
Besides the development of cytokine storm and exhaustion of immune cells, the clinical outcomes of SARS-CoV-2 infection are also dependent on multiple factors, such as age; clinical morbidities, including metabolic disorders like obesity, diabetes, cardiovascular and liver disease, and other conditions, such as pregnancy and cancer [
38,
39,
40]. Notably, the expression of ACE2 receptors tend to increase with increasing age, and the majority of cancer is diagnosed at near to 60 years of age, marking cancer patients as more prone to SARS-CoV-2 infection. Consequently, that led to adverse clinical outcomes [
41]. In addition to changes in the ACE2 receptor, immune functions in cancer patients are compromised. In fact, there is an increase in the expression of immunosuppressive cytokines and markers, such as PD1/PD-L1 on immune cells, which dampened the immune system and augmented the probability of viral infection [
42]. Moreover, specific cytokines, such as IL17, secreted by Th17 cells in response to viral infection, have been shown to play a central role in virus pathogenesis through regulating the program cell death, cytokine storm, and lung cancer progression through VEGF (vascular endothelial growth factor) expression stimulation [
40,
43,
44]. Therefore, therapies targeting such cytokines and anti-neoplastic agents might ideally improve the clinical efficacy of SARS-CoV-2-infected cancer patients [
45].
5. Immune Responses of Asymptomatic Patients with SARS-CoV-2 Infection
Immune responses and clinical features of asymptomatic individuals with COVID-19 have not been well defined. These individuals comprise approximately 40–45% of the infected population and can silently spread the virus to others. The absence of symptoms in these infected patients does not mean that they are away from ultimate mortality risk, as they might have viral load equivalent to symptomatic patients. Therefore, more investigations are needed to understand the significant changes in disease symptoms, viral load, proper examination, and immune responses; all this knowledge might be useful to develop directed therapy to prevent the spread of this infection. Compared to asymptomatic people, recovered individuals (virus-negative/antibody-positive) can safely interact with susceptible and infected individuals [
46]. Long Q and colleagues studied clinical features and immune responses of 37 asymptomatic individuals (20.8%), identified in a group of total 178 Real time polymerase chain reaction confirmed SARS-COV-2-positive people in the Wanzhou District of China [
47]. However, this might not be an accurate assessment of the percentage of asymptomatic infections in the general population, since asymptomatic infections were identified from those who are at high risk of infection and not from a random sample of people. Therefore, the proportion of asymptomatic infections needs to be determined through population screening [
48].
According to Long Q et al., the median duration of virus-shedding time in asymptomatic individuals was significantly longer than the symptomatic group. Moreover, the levels of virus-specific immunoglobulin (IgG) and levels of 18 anti-inflammatory cytokines were significantly lower in the asymptomatic group [
47]. Previous studies have shown that circulating antibodies against SARS-CoV or MERS-CoV could remain for nearly 3 years or longer [
49,
50]. Several studies have reported that most SARS-CoV-2 convalescent individuals have detectable neutralizing antibodies, which correlate with the numbers of virus-specific T cells [
51,
52]. Additional longitudinal serological studies profiling more symptomatic and asymptomatic individuals are urgently needed to determine the duration of antibody-mediated immunity.
This entry is adapted from the peer-reviewed paper 10.3390/biology9090243