Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Cancer is one of the major diseases that endanger human health. Bacteria is a novel drug delivery system that has shown great potential in cancer therapy because of its tumor-targeting, oncolytic, and immunomodulatory properties.
- cancer therapy
- bacteria
- drug carrier
- drug-loading strategies
1. Introduction
Cancer is one of the major diseases that endanger human life and health. According to a report by the International Agency for Research on Cancer, there were about 19.3 million new cases and 10 million deaths of cancer patients worldwide in 2020 [1]. However, the current treatment methods, such as chemotherapy and radiotherapy, are accompanied by serious side effects, such as gastrointestinal toxicity, bone marrow suppression, alopecia, and so on [2]. The occurrence of these side effects is mainly related to the non-targeted distribution of drugs. Therefore, the development of effective tumor-targeted delivery systems is one of the main development directions of anticancer drug delivery systems. Nowadays, widely studied drug delivery systems, such as liposomes, micelles, and nanoparticles (NPs), have been shown to improve the anticancer effects of drugs, but still have some shortcomings, including low biocompatibility, high off-target effects, and rapid clearance in the blood. Dai et al. quantified the cancer cell targeting efficiency of nanoparticles in solid tumors, showing that only 0.7% of intravenous NPs were delivered to solid tumors, and only 0.0014% of NPs were delivered to cancer cells [3]. Most NPs are either captured in the extracellular matrix or absorbed by perivascular tumor-associated macrophages. Therefore, it is urgent to develop new and effective drug delivery systems to overcome the existing limitations.
In recent years, bacterial-based biocarriers have received widespread attention in the field of cancer therapy due to their obvious tumor targeting, oncolytic, and immunomodulatory properties. The use of bacteria in cancer treatment can be traced back to the 19th century. Coley et al. successfully achieved tumor reduction by injecting Streptococcus into cancer patients [4]. Currently, most of the bacteria used in cancer therapy are obligate and facultative anaerobic bacteria, such as Salmonella typhimurium (S. typhimurium), Listeria, Escherichia coli (E. coli), Bifidobacterium, Clostridium, and so on [5][6][7][8][9]. Some self-mineralizing and magneto-aerotactic bacteria have also been tried for oncology treatment [10][11]. Researchers have tried to carry anticancer substances, such as drug molecules, immune factors, and nucleic acids, on the surface of bacteria through chemical bonds or electrostatic adsorption, or inside bacteria through incubation or gene editing. Drug-loaded bacteria will release drugs after reaching the tumor site, thereby achieving tumor-targeted drug delivery. Under the combined action of drugs and bacteria, cancer cells undergo autophagy and apoptosis, while the body’s immune system will be activated, to achieve multiple pathways to inhibit tumor growth and diffusion [12][13]. Unlike general delivery systems that can only be passively transported to the surface of the tumor, flagellated bacteria can also move autonomously, which can make up for the lack of permeability of tumor tissue in existing drug delivery systems.
It is worth noting that with the development of gene editing technology, the safety and feasibility of modern bacterial therapy have been greatly improved. For example, the knockout of toxic genes can significantly reduce the toxicity of engineered bacteria compared with wild strains [14][15], and the knockout of nutrient-producing genes can make auxotrophic engineered bacteria colonize far more in tumors than in normal tissues [16][17].
2. The Advantages and Mechanisms of Bacteria for Cancer Therapy
2.1. Tumor-Targeting of Bacterial Carriers
As a new type of anticancer drug carrier, bacteria will preferentially accumulate in tumors after entering the human body. Compared with healthy tissues, the accumulation of bacteria in tumor tissues is more than 1000 times higher [15]. Unlike liposomes, micelles, and NPs which can only passively target tumors by bloodstream transport, the bacteria have both passive and active targeting mechanisms and can actively penetrate deep into the tumor tissue [18][19]. The generation of the targeting property can be related to the suitable tumor microenvironment (TME) and the properties of bacteria. In addition, various modification methods can further improve the tumor-targeting ability of bacteria (Figure 1).
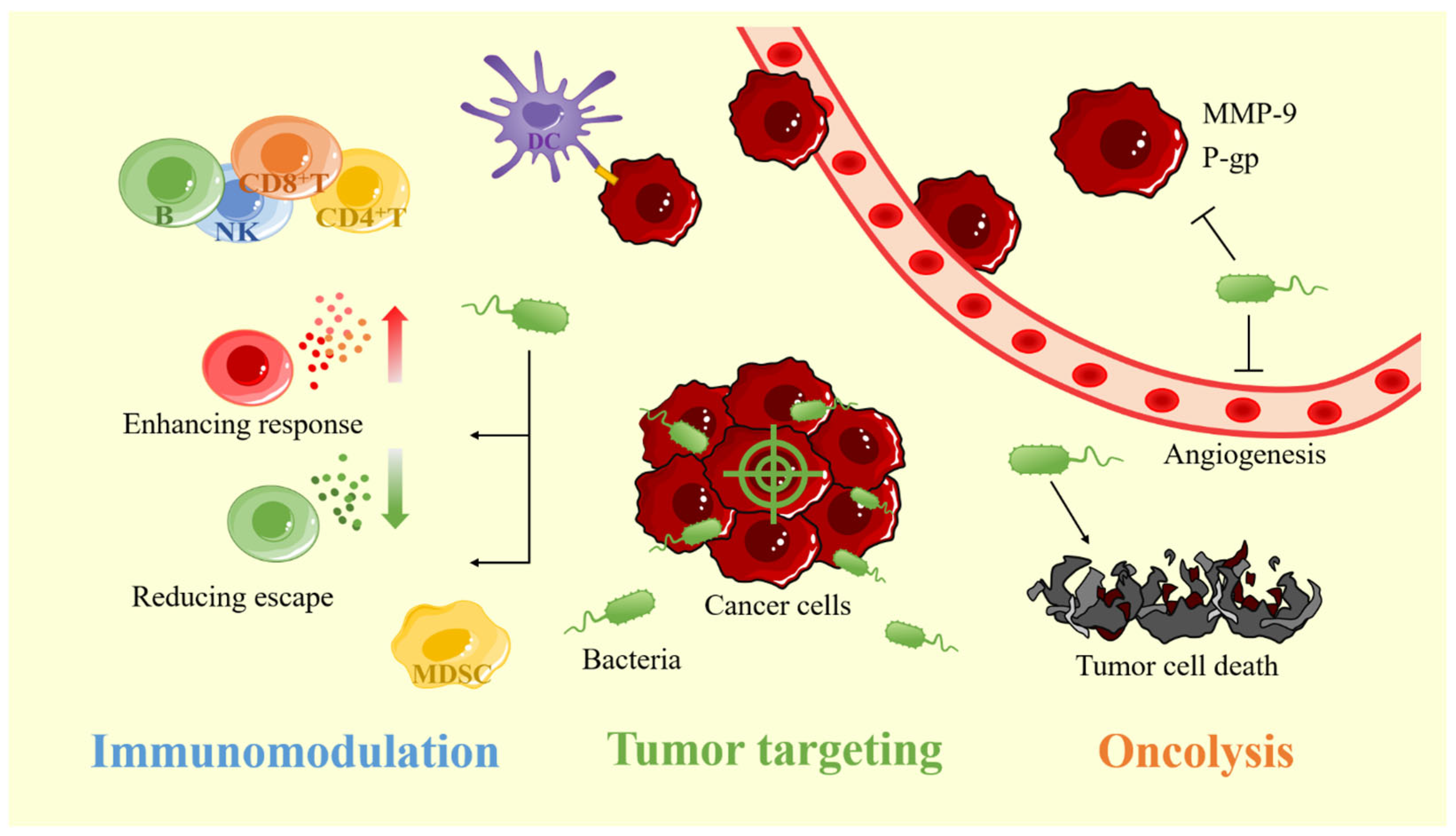
Figure 1. The advantages of bacterial carriers for cancer therapy include tumor-targeting, oncolytic and, immunomodulatory properties. MMP-9: matrix metalloproteinase-9; P-gp: p-glycoprotein.
2.1.1. Suitable TME for Bacterial Survival
The external reasons for the tumor targeting of bacteria can be roughly divided into three points. Firstly, the hypoxia TME attracts obligate and facultative anaerobic bacteria to tumor tissue. Due to the rapid proliferation of tumor cells and incomplete vascular development, the supply of oxygen in solid tumors is insufficient, which eventually leads to the existence of hypoxic areas in tumors [20]. This is a necessary condition for the survival and reproduction of obligate and facultative anaerobic bacteria in tumors. Moreover, with further penetration of the tumor tissue, hypoxia tends to be more severe. This is very unfavorable for conventional treatment but is more conducive for bacterial tropism and depth of penetration [21][22]. Secondly, the rich nutrients in the tumor tissue are another important reason for attracting bacterial colonization. Kasinskas et al. demonstrated that aspartic acid, serine, ribose, and galactose in the tumor can help Salmonella chemotaxis [23]. Song et al. demonstrated that clusterin is one of the key biochemical molecules in E. coli chemotaxis of lung cancer cells [24]. Lastly, the Immunosuppressive properties of tumors benefit bacterial colonization [25]. Bacteria that colonize tumor sites in the early stages can avoid clearance by the immune system, while bacteria that travel to normal tissues will be cleared by the immune system. Therefore, the concentration of bacteria in the tumor site is often much higher than in other sites, and the bacteria do not colonize the non-tumor-related hypoxic or inflammatory lesions [26][27][28][29].
2.1.2. The Chemotaxis Properties of Bacteria
Some of the properties of bacteria can also help them to colonize tumors. It has been proposed that some chemical-specific receptors on the bacteria may sense chemicals secreted by cancer cells [19][23]. Kasinskas et al. proved that chemical receptors have an important effect on the tumor tropism of bacteria by knocking out different chemical receptors on the surface of Salmonella [23]. More interestingly, the accumulation of bacteria in different parts of the tumor can be controlled by selectively eliminating chemical receptor genes. In addition, some bacteria have special tendency properties. For example, magnetotactic bacteria have a tendency to a specific magnetic field [30]. By giving an auxiliary magnetic field in vitro, it can induce magneto-aerotactic bacteria to migrate to the tumor site.
There are two opposing views on the relationship between bacterial motility and tumor tropism. One side believes that motility is essential for the effective distribution and accumulation of bacteria in tumor tissues. Studies by Kasinskas et al. and Toley et al. both used in vitro models to demonstrate that motility is essential for the effective distribution of bacteria in tumors [23][31]. On the contrary, Stritzker et al. found that chemotaxis and motility did not seem to help the bacteria colonize the tumor by intravenously injecting E. coli and Salmonella strains into the BALB/c mice with 4T1 breast cancer [32]. However, this study only analyzed the distribution of bacteria within the tumor at 48h after injection. Later, Ganai et al. conducted a longer experimental study on the same animal model [33]. Interestingly, the results showed that the bacteria began to migrate from the tumor edge to the central core after 48 h, and began to move closer to the tumor transition zone after 96 h. This may indicate that the experiment of Stritzker et al. still has some flaws and, if they could do longer tests, they may give a different conclusion.
It is undeniable that the movement of bacteria appears to be very insignificant compared to the speed of blood flow [34]. Therefore, bacteria entering the animal’s body are mainly transported passively by blood flow to the tumor site. But, upon reaching the tumor, the motility of the bacteria plays a crucial role in helping the bacteria penetrate deeper into the core of the tumor. When bacteria penetrate the tumor tissue, they will multiply in the tumor. Combined with the previously described immune clearance phase in normal tissues, this results in a much larger number of bacteria at the tumor site than in normal tissues.
2.1.3. Tumor-Targeting Modifications of Bacterial Carriers
Gene editing and surface modification can further improve bacteria’s tumor-targeting ability. With the use of gene editing technology, it is possible to design modified auxotrophic bacteria corresponding to some specific purines, amino acids, and other nutrients within the tumor. Zhao et al. designed a leucine–arginine auxotroph S. typhimurium [35]. The engineered bacteria can only survive in tumors but not in normal tissues. In terms of surface modification, modifying tumor-homing peptides or tumor antibodies on the surface of bacteria can lead to better tumor-targeting ability. Park et al. improved the tumor tropism of bacteria by modifying an arginine–glycine–aspartate peptide on the outer membrane protein of S. typhimurium [36]. Massa et al. demonstrated that the tumor specificity of Salmonella can be significantly improved by surface-expressed antibodies against tumor-associated antigens [37].
2.2. Immunomodulatory Effects of Bacterial Carriers
Cancer patients cannot clear cancer cells through their immune system, because cancer cells can develop multiple pathways to avoid being cleared by the immune system [38]. It has been found that bacteria inside the tumor can alter the immunosuppressive TME and stimulate the host immune system, thus enhancing the body’s immune system to clear the cancer cells (Figure 1).
2.2.1. Weak Antitumor Immunity
The reasons for the inability of the anticancer immune response to eliminate tumors can be broadly classified as follows:
-
Tumor cells themselves have developed specialized mechanisms to suppress immune responses, including downregulation of tumor antigen and major histocompatibility complex (MHC) class I expression [39][40], high expression of programmed death receptor-ligand 1 (PD-L1) to prevent T cell activation [41], and expression of various immunosuppressive cytokines and chemokines by themselves or induced tumor-infiltrating immune cells [38][42].
-
The patient’s immune function is so weak that the growth rate of tumor cells exceeds the clearance rate of the immune system [46]. The combination of these factors makes it difficult for the body to rely on its immune system to remove tumor cells, thus allowing the tumor to grow and spread.
2.2.2. Bacteria Activate the Immune System
Bacteria at the tumor site can stimulate the immune system to produce a series of anticancer immune responses is another advantage of bacterial carriers in cancer therapy. This is because bacteria carry a large number of antigens such as lipopolysaccharide and flagellin, which can bind to toll-like receptors and, thus, trigger a series of cellular signaling events [47][48][49]. The mechanisms by which bacteria activate anticancer immunity in the body are complex and interact with each other. For the sake of description, these mechanisms are roughly divided into two parts: promotion of anticancer immune response and reduction of tumor immune escape (Figure 2).
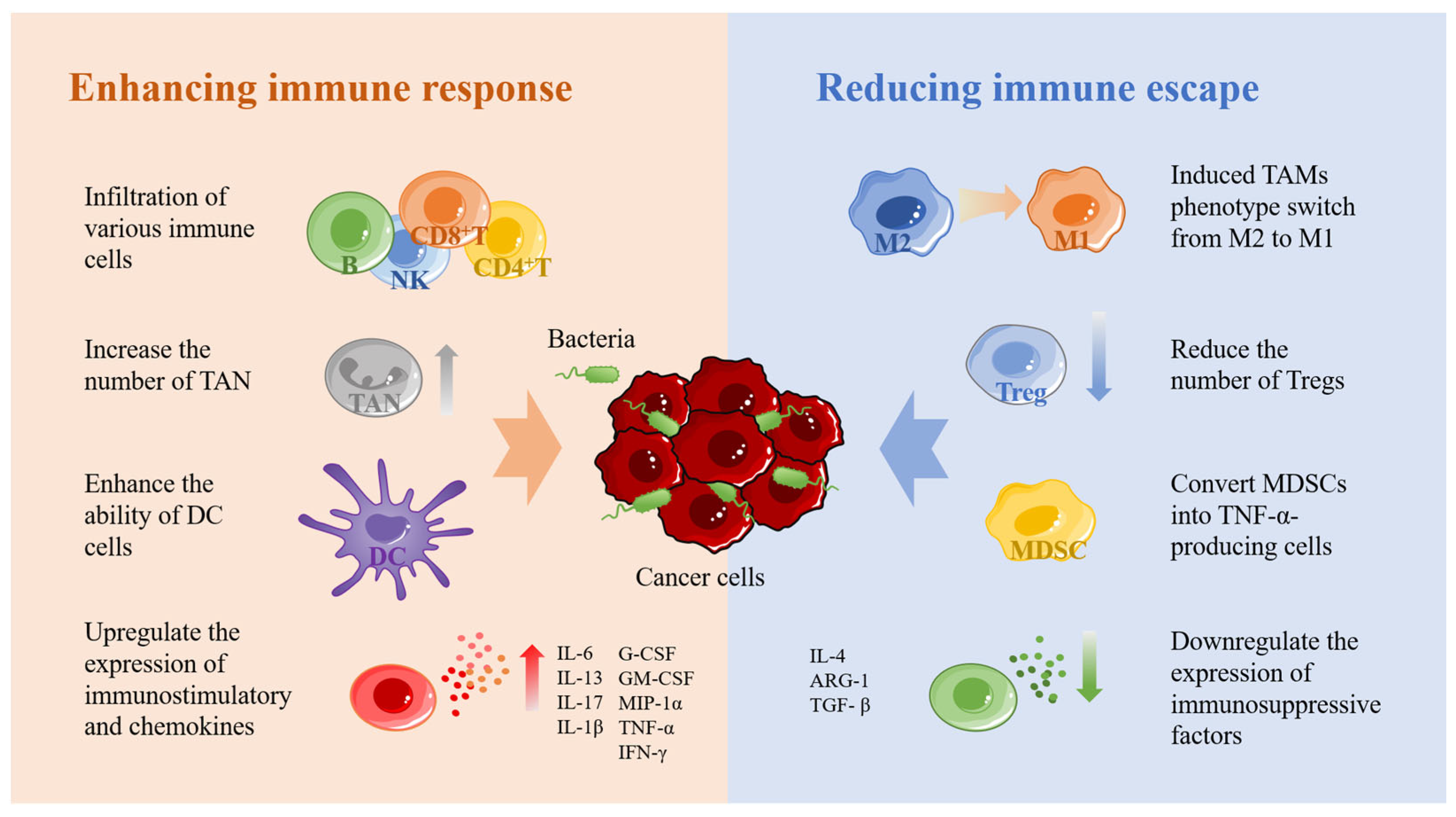
Figure 2. The immune stimulation mechanism of bacteria. Bacteria can recruit a large number of immune cells and produce a series of immune responses after colonization in tumors. NK: natural killer cells; TAN: tumor-associated neutrophils; DC: dendritic cell; M1: M1-like macrophage; M2: M2-like macrophage. Tregs: regulatory T cells; MDSC: myeloid-derived suppressor cells; IL-6: interleukin-6; IL-13: interleukin-13; IL-17: interleukin-17; IL-1β: interleukin-1β; G-CSF: granulocyte colony-stimulating factor; GM-CSF: granulocyte-macrophage colony-stimulating factor; MIP-1α: macrophage inflammatory protein-1α; TNF-α: tumor necrosis factor- α; IFN-γ: interferon-γ; IL-4: interleukin-4; ARG-1: arginase-1; TGF-β: transforming growth factor-β.
In enhancing the anticancer immune response, the recruitment of immune cells to reach the tumor site is the most fundamental step. Several studies have demonstrated that bacteria at the tumor site can induce a large number of immune cells to accumulate toward the tumor, including macrophages, NK cells, B cells, CD4+ T cells, CD8+ T cells, and so on [50][51][52]. When a large number of immune cells reach the tumor site, a series of anticancer immune responses are triggered. Secondly, bacteria can increase the number of TANs and, thus, enhance the innate immune response [53]. However, neutrophils show two sides in the development of tumors [54][55][56]. TAN1 exhibits anticancer activity through direct cytotoxic effects and activation of adaptive immune responses, while TAN2 exhibits tumor-promoting activity by promoting tumor cell proliferation, migration, and invasion, stimulating angiogenesis and mediating immunosuppression. Furthermore, bacteria promote gap junctions between DCs and cancer cells by upregulating connexin 43 to enhance the ability of dendritic cells to present tumor antigens [57][58]. This process leads to the secretion of large amounts of the proinflammatory cytokine IL-1β by DCs and subsequent activation of CD8+ T cells. Lastly, bacteria were found to activate the NF-κB pathway, which in turn upregulates the expression of various immunostimulatory and chemokines, including IL-6, IL-13, IL-17, IL-1β, G-CSF, GM-CSF, MIP-1α, TNF-α, and IFN-γ [59][60][61][62].
Reducing tumor escape is another important means to activate antitumor immunity. As mentioned above, tumor immune escape is associated with a large number of immunosuppressive cells, including TAMs, Treg, and MDSCs, which protect tumor cells in various ways to treat the activation of cytotoxic T cells [62]. Among them, TAMs are usually divided into two opposite subtypes, including M1 macrophages that exert antitumor activity and M2 macrophages that inhibit T-cell-mediated antitumor immune response. Both M1 and M2 macrophages have a high degree of plasticity, so they can be transformed into each other when the tumor microenvironment changes or therapeutic interventions [63]. It was found that S. typhimurium expressing flagellin promoted the M2–M1 transition of macrophages and increased the level of nitric oxide in tumors through the synergistic effect with TLR4 and TLR5 signaling pathways [64]. In addition, flagellin stimulates NK cells to produce IFN-γ, which can also promote the conversion of M2 to M1 [65]. Treg cells and MDSCs are the other two important immunosuppressive cells. Treg cells can inhibit the costimulatory signal of CD80 and CD86 expressed by dendritic cells, consume IL-2, secrete inhibitory cytokines, and directly kill effector T cells [66]. MDSCs play an important role in tumor angiogenesis, drug resistance and tumor metastasis [67]. Studies have found that some bacteria can effectively reduce Treg and DMSCs in tumor tissues. For example, attenuated Salmonella vaccine has been shown to induce immunosuppressive myeloid-derived suppressor cells to transform into TNF-αsecreting cells with neutrophil characteristics and significantly reduce Treg cells [68][69][70]. Secondly, Listeria was found to be delivered to metastatic and primary tumors by infecting DMSCs, significantly reduce the number of MDSC in blood and primary tumors, and transform the remaining MDSC subsets into an immunostimulatory phenotype that produces IL-12 [71]. In addition, bacteria downregulate the expression of immunosuppressive factors such as IL-4, ARG-1, and TGF-β [62][72].
2.3. Oncolysis of Bacterial Carriers
In addition to activating the immune system to kill cancer cells, bacteria colonizing tumors also have a certain anticancer effect, which can help drugs better play their anticancer role. This oncolytic activity can be achieved through a variety of pathways, including the induction of tumor cell death, inhibition of tumor angiogenesis, inhibition of tumor metastasis, and reduction of tumor drug resistance (Figure 1).
2.3.1. Induction of Tumor Cell Death
Bacteria induce cancer cell death through multiple pathways, including the induction of apoptosis, the release of bacterial toxins, and competition for nutrients. For example, Listeria can induce tumor cell apoptosis by activating nicotinamide adenine dinucleotide phosphate (NADPH) oxidase and increasing intracellular calcium levels to increase ROS levels in tumors [73]. Salmonella induces autophagy and caspase-mediated apoptosis in tumor cells by downregulating the AKT/mTOR pathway [74], and metabolizes nitrate to nitrite via nitrate reductase and further converts it to nitric oxide in tumors to induce tumor cell apoptosis [75]. Secondly, bacteria can release toxins to kill tumor cells. For example, Colicins have anticancer activity against breast cancer, colon cancer, bone cancer, and other human tumor cell lines [76]. However, bacterial toxins are a double-edged sword. Strong toxicity may lead to damage to other normal tissues while showing good tumor killing. Therefore, it is necessary to select the appropriate intensity of toxicity as well as the dose. Lastly, the growth and reproduction of bacteria at the tumor site can consume a large number of nutrients, and by competing with tumor cells for nutrients it can promote apoptosis of some tumor cells [77].
2.3.2. Inhibition of Tumor Angiogenesis
Tumor growth is closely linked to tumor angiogenesis. The rapid growth of tumor cells requires a large amount of oxygen and nutrients; therefore, a large number of blood vessels are generated inside the tumor to transport the materials required by the tumor cells. If angiogenesis fails, the lack of oxygen and nutrients limits the tumor diameter to 2–3 mm [78]. The hypoxic TME can stimulate the tumor to produce some adaptive responses. At this time, the tumor cells will activate the hypoxia-inducible factor (HIF) signal and upregulate the expression of vascular endothelial growth factor (VEGF) and other proangiogenic factors, thereby increasing tumor angiogenesis [79][80]. Salmonella was found to downregulate HIF-1α expression via downregulation of the AKT/mTOR pathway, which in turn inhibited tumor VEGF expression and angiogenic signaling [81].
2.3.3. Inhibition of Tumor Metastasis
The metastasis of malignant tumors is frequently the main cause of tumor treatment failure, and the degradation of extracellular matrix caused by matrix metalloproteinase-9 (MMP-9) plays a key role in this process. Cancer cells can break through the physical barrier of the extracellular matrix by influencing host cells to secrete MMP-9 to degrade multiple collagen proteins in the basement membrane to metastasize and invade other tissues [82]. Tsao et al. demonstrated in a mouse model of melanoma and lung cancer that Salmonella inhibited MMP-9 expression by downregulating the AKT/mTOR signaling pathway, resulting in the inhibition of tumor cell migration and reduction of nodule production in vivo [83].
2.3.4. Reduction of Tumor Drug Resistance
Reducing the drug resistance of tumor cells is also one of how bacteria achieve oncolysis. p-glycoprotein (P-gp), also called multidrug resistance protein, is a protein that pumps certain intracellular chemicals out of the cell and reduces their concentration in the cell for cell protection [84]. The P-gp is also distributed on the surface of tumor cells, which allows the chemotherapeutic drugs inside the tumor cells to be excreted extracellularly, thus causing the tumor cells to develop drug resistance. In mouse melanoma and mammary tumor models, Salmonella reduced the expression of P-gp by inhibiting the expression levels of phosph-protein kinase B, phosph-mammalian targets of rapamycin, and phosphate-p70 ribosomal s6 kinase in tumor cells., thus improving the sensitivity of tumors to chemotherapy [85].
This entry is adapted from the peer-reviewed paper 10.3390/pharmaceutics15092214
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249.
- Schirrmacher, V. From chemotherapy to biological therapy: A review of novel concepts to reduce the side effects of systemic cancer treatment (Review). Int. J. Oncol. 2019, 54, 407–419.
- Dai, Q.; Wilhelm, S.; Ding, D.; Syed, A.M.; Sindhwani, S.; Zhang, Y.; Chen, Y.Y.; MacMillan, P.; Chan, W.C.W. Quantifying the Ligand-Coated Nanoparticle Delivery to Cancer Cells in Solid Tumors. ACS Nano 2018, 12, 8423–8435.
- McCarthy, E.F. The toxins of William B. Coley and the treatment of bone and soft-tissue sarcomas. Iowa Orthop. J. 2006, 26, 154–158.
- Chorobik, P.; Czaplicki, D.; Ossysek, K.; Bereta, J. Salmonella and cancer: From pathogens to therapeutics. Acta Biochim. Pol. 2013, 60, 285–297.
- Liu, Y.; Lu, Y.; Ning, B.; Su, X.; Yang, B.; Dong, H.; Yin, B.; Pang, Z.; Shen, S. Intravenous Delivery of Living Listeria monocytogenes Elicits Gasdmermin-Dependent Tumor Pyroptosis and Motivates Anti-Tumor Immune Response. ACS Nano 2022, 16, 4102–4115.
- Yu, X.; Lin, C.; Yu, J.; Qi, Q.; Wang, Q. Bioengineered Escherichia coli Nissle 1917 for tumour-targeting therapy. Microb. Biotechnol. 2020, 13, 629–636.
- Fahmy, C.A.; Gamal-Eldeen, A.M.; El-Hussieny, E.A.; Raafat, B.M.; Mehanna, N.S.; Talaat, R.M.; Shaaban, M.T. Bifidobacterium longum Suppresses Murine Colorectal Cancer through the Modulation of oncomiRs and Tumor Suppressor miRNAs. Nutr. Cancer 2019, 71, 688–700.
- Wang, Y.; Liu, Y.; Zhu, H.; Wang, D.; Wang, S.; Xu, X.; Yu, N.; Feng, J.; Zou, J.; Wang, X.; et al. Bacteriolytic therapy with Clostridium ghonii for experimental solid tumors. Biochem. Biophys. Res. Commun. 2022, 634, 114–121.
- Chen, Q.W.; Liu, X.H.; Fan, J.X.; Peng, S.Y.; Wang, J.W.; Wang, X.N.; Zhang, C.; Liu, C.J.; Zhang, X.Z. Self-Mineralized Photothermal Bacteria Hybridizing with Mitochondria-Targeted Metal-Organic Frameworks for Augmenting Photothermal Tumor Therapy. Adv. Funct. Mater. 2020, 30, 1909806.
- Felfoul, O.; Mohammadi, M.; Taherkhani, S.; de Lanauze, D.; Zhong Xu, Y.; Loghin, D.; Essa, S.; Jancik, S.; Houle, D.; Lafleur, M.; et al. Magneto-aerotactic bacteria deliver drug-containing nanoliposomes to tumour hypoxic regions. Nat. Nanotechnol. 2016, 11, 941–947.
- Yaghoubi, A.; Khazaei, M.; Jalili, S.; Hasanian, S.M.; Avan, A.; Soleimanpour, S.; Cho, W.C. Bacteria as a double-action sword in cancer. Biochim. Biophys. Acta Rev. Cancer 2020, 1874, 188388.
- Guo, Y.; Chen, Y.; Liu, X.; Min, J.J.; Tan, W.; Zheng, J.H. Targeted cancer immunotherapy with genetically engineered oncolytic Salmonella typhimurium. Cancer Lett. 2020, 469, 102–110.
- Jiang, S.N.; Park, S.H.; Lee, H.J.; Zheng, J.H.; Kim, H.S.; Bom, H.S.; Hong, Y.; Szardenings, M.; Shin, M.G.; Kim, S.C.; et al. Engineering of bacteria for the visualization of targeted delivery of a cytolytic anticancer agent. Mol. Ther. 2013, 21, 1985–1995.
- Liang, K.; Liu, Q.; Li, P.; Luo, H.; Wang, H.; Kong, Q. Genetically engineered Salmonella Typhimurium: Recent advances in cancer therapy. Cancer Lett. 2019, 448, 168–181.
- Clairmont, C.; Lee, K.C.; Pike, J.; Ittensohn, M.; Low, K.B.; Pawelek, J.; Bermudes, D.; Brecher, S.M.; Margitich, D.; Turnier, J.; et al. Biodistribution and genetic stability of the novel antitumor agent VNP20009, a genetically modified strain of Salmonella typhimurium. J. Infect. Dis. 2000, 181, 1996–2002.
- Luo, X.; Li, Z.; Lin, S.; Le, T.; Ittensohn, M.; Bermudes, D.; Runyab, J.D.; Shen, S.Y.; Chen, J.; King, I.C.; et al. Antitumor effect of VNP20009, an attenuated Salmonella, in murine tumor models. Oncol. Res. 2001, 12, 501–508.
- Leschner, S.; Westphal, K.; Dietrich, N.; Viegas, N.; Jablonska, J.; Lyszkiewicz, M.; Lienenklaus, S.; Falk, W.; Gekara, N.; Loessner, H.; et al. Tumor invasion of Salmonella enterica serovar Typhimurium is accompanied by strong hemorrhage promoted by TNF-alpha. PLoS ONE 2009, 4, e6692.
- Kasinskas, R.W.; Forbes, N.S. Salmonella typhimurium specifically chemotax and proliferate in heterogeneous tumor tissue in vitro. Biotechnol. Bioeng. 2006, 94, 710–721.
- Vaupel, P.; Mayer, A. Tumor Hypoxia: Causative Mechanisms, Microregional Heterogeneities, and the Role of Tissue-Based Hypoxia Markers. Adv. Exp. Med. Biol. 2016, 923, 77–86.
- Muz, B.; de la Puente, P.; Azab, F.; Azab, A.K. The role of hypoxia in cancer progression, angiogenesis, metastasis, and resistance to therapy. Hypoxia 2015, 3, 83–92.
- Graham, K.; Unger, E. Overcoming tumor hypoxia as a barrier to radiotherapy, chemotherapy and immunotherapy in cancer treatment. Int. J. Nanomed. 2018, 13, 6049–6058.
- Kasinskas, R.W.; Forbes, N.S. Salmonella typhimurium lacking ribose chemoreceptors localize in tumor quiescence and induce apoptosis. Cancer Res. 2007, 67, 3201–3209.
- Song, J.; Zhang, Y.; Zhang, C.; Du, X.; Guo, Z.; Kuang, Y.; Wang, Y.; Wu, P.; Zou, K.; Zou, L.; et al. A microfluidic device for studying chemotaxis mechanism of bacterial cancer targeting. Sci. Rep. 2018, 8, 6394.
- Bennaceur, K.; Chapman, J.A.; Touraine, J.L.; Portoukalian, J. Immunosuppressive networks in the tumour environment and their effect in dendritic cells. Biochim. Biophys. Acta 2009, 1795, 16–24.
- Staedtke, V.; Bai, R.Y.; Sun, W.; Huang, J.; Kibler, K.K.; Tyler, B.M.; Gallia, G.L.; Kinzler, K.; Vogelstein, B.; Zhou, S.; et al. Clostridium novyi-NT can cause regression of orthotopically implanted glioblastomas in rats. Oncotarget 2015, 6, 5536–5546.
- Zhou, S.; Gravekamp, C.; Bermudes, D.; Liu, K. Tumour-targeting bacteria engineered to fight cancer. Nat. Rev. Cancer 2018, 18, 727–743.
- Yu, Y.A.; Zhang, Q.; Szalay, A.A. Establishment and characterization of conditions required for tumor colonization by intravenously delivered bacteria. Biotechnol. Bioeng. 2008, 100, 567–578.
- Diaz, L.A., Jr.; Cheong, I.; Foss, C.A.; Zhang, X.; Peters, B.A.; Agrawal, N.; Bettegowda, C.; Karim, B.; Liu, G.; Khan, K.; et al. Pharmacologic and toxicologic evaluation of C. novyi-NT spores. Toxicol. Sci. 2005, 88, 562–575.
- Kotakadi, S.M.; Borelli, D.P.R.; Nannepaga, J.S. Therapeutic Applications of Magnetotactic Bacteria and Magnetosomes: A Review Emphasizing on the Cancer Treatment. Front. Bioeng. Biotechnol. 2022, 10, 789016.
- Toley, B.J.; Forbes, N.S. Motility is critical for effective distribution and accumulation of bacteria in tumor tissue. Integr. Biol. 2012, 4, 165–176.
- Stritzker, J.; Weibel, S.; Seubert, C.; Gotz, A.; Tresch, A.; van Rooijen, N.; Oelschlaeger, T.A.; Hill, P.J.; Gentschev, I.; Szalay, A.A. Enterobacterial tumor colonization in mice depends on bacterial metabolism and macrophages but is independent of chemotaxis and motility. Int. J. Med. Microbiol. 2010, 300, 449–456.
- Ganai, S.; Arenas, R.B.; Sauer, J.P.; Bentley, B.; Forbes, N.S. In tumors Salmonella migrate away from vasculature toward the transition zone and induce apoptosis. Cancer Gene Ther. 2011, 18, 457–466.
- Wadhwa, N.; Berg, H.C. Bacterial motility: Machinery and mechanisms. Nat. Rev. Microbiol. 2022, 20, 161–173.
- Zhao, M.; Yang, M.; Ma, H.; Li, X.; Tan, X.; Li, S.; Yang, Z.; Hoffman, R.M. Targeted therapy with a Salmonella typhimurium leucine-arginine auxotroph cures orthotopic human breast tumors in nude mice. Cancer Res. 2006, 66, 7647–7652.
- Park, S.H.; Zheng, J.H.; Nguyen, V.H.; Jiang, S.N.; Kim, D.Y.; Szardenings, M.; Min, J.H.; Hong, Y.; Choy, H.E.; Min, J.J. RGD Peptide Cell-Surface Display Enhances the Targeting and Therapeutic Efficacy of Attenuated Salmonella-mediated Cancer Therapy. Theranostics 2016, 6, 1672–1682.
- Massa, P.E.; Paniccia, A.; Monegal, A.; de Marco, A.; Rescigno, M. Salmonella engineered to express CD20-targeting antibodies and a drug-converting enzyme can eradicate human lymphomas. Blood 2013, 122, 705–714.
- Yaguchi, T.; Sumimoto, H.; Kudo-Saito, C.; Tsukamoto, N.; Ueda, R.; Iwata-Kajihara, T.; Nishio, H.; Kawamura, N.; Kawakami, Y. The mechanisms of cancer immunoescape and development of overcoming strategies. Int. J. Hematol. 2011, 93, 294–300.
- Uyttenhove, C.; Maryanski, J.; Boon, T. Escape of mouse mastocytoma P815 after nearly complete rejection is due to antigen-loss variants rather than immunosuppression. J. Exp. Med. 1983, 157, 1040–1052.
- Cornel, A.M.; Mimpen, I.L.; Nierkens, S. MHC Class I Downregulation in Cancer: Underlying Mechanisms and Potential Targets for Cancer Immunotherapy. Cancers 2020, 12, 1760.
- Jiang, X.; Wang, J.; Deng, X.; Xiong, F.; Ge, J.; Xiang, B.; Wu, X.; Ma, J.; Zhou, M.; Li, X.; et al. Role of the tumor microenvironment in PD-L1/PD-1-mediated tumor immune escape. Mol. Cancer 2019, 18, 10.
- Garner, H.; de Visser, K.E. Immune crosstalk in cancer progression and metastatic spread: A complex conversation. Nat. Rev. Immunol. 2020, 20, 483–497.
- Tanaka, A.; Sakaguchi, S. Targeting Treg cells in cancer immunotherapy. Eur. J. Immunol. 2019, 49, 1140–1146.
- Weber, R.; Groth, C.; Lasser, S.; Arkhypov, I.; Petrova, V.; Altevogt, P.; Utikal, J.; Umansky, V. IL-6 as a major regulator of MDSC activity and possible target for cancer immunotherapy. Cell Immunol. 2021, 359, 104254.
- Burstyn-Cohen, T.; Maimon, A. TAM receptors, Phosphatidylserine, inflammation, and Cancer. Cell Commun. Signal 2019, 17, 156.
- Hinshaw, D.C.; Shevde, L.A. The Tumor Microenvironment Innately Modulates Cancer Progression. Cancer Res. 2019, 79, 4557–4566.
- Kim, J.E.; Phan, T.X.; Nguyen, V.H.; Dinh-Vu, H.V.; Zheng, J.H.; Yun, M.; Park, S.G.; Hong, Y.; Choy, H.E.; Szardenings, M.; et al. Salmonella typhimurium Suppresses Tumor Growth via the Pro-Inflammatory Cytokine Interleukin-1beta. Theranostics 2015, 5, 1328–1342.
- Cai, Z.; Sanchez, A.; Shi, Z.; Zhang, T.; Liu, M.; Zhang, D. Activation of Toll-like receptor 5 on breast cancer cells by flagellin suppresses cell proliferation and tumor growth. Cancer Res. 2011, 71, 2466–2475.
- Guo, L.; Ding, J.; Zhou, W. Harnessing bacteria for tumor therapy: Current advances and challenges. Chin. Chem. Lett. 2023, 108557.
- Grille, S.; Moreno, M.; Bascuas, T.; Marques, J.M.; Munoz, N.; Lens, D.; Chabalgoity, J.A. Salmonella enterica serovar Typhimurium immunotherapy for B-cell lymphoma induces broad anti-tumour immunity with therapeutic effect. Immunology 2014, 143, 428–437.
- Lee, C.H.; Hsieh, J.L.; Wu, C.L.; Hsu, P.Y.; Shiau, A.L. T cell augments the antitumor activity of tumor-targeting Salmonella. Appl. Microbiol. Biotechnol. 2011, 90, 1381–1388.
- Lee, C.H.; Wu, C.L.; Shiau, A.L. Toll-like receptor 4 mediates an antitumor host response induced by Salmonella choleraesuis. Clin. Cancer Res. 2008, 14, 1905–1912.
- Li, C.X.; Yu, B.; Shi, L.; Geng, W.; Lin, Q.B.; Ling, C.C.; Yang, M.; Ng, K.T.; Huang, J.D.; Man, K. ‘Obligate’ anaerobic Salmonella strain YB1 suppresses liver tumor growth and metastasis in nude mice. Oncol. Lett. 2017, 13, 177–183.
- Jaillon, S.; Ponzetta, A.; Di Mitri, D.; Santoni, A.; Bonecchi, R.; Mantovani, A. Neutrophil diversity and plasticity in tumour progression and therapy. Nat. Rev. Cancer 2020, 20, 485–503.
- Hedrick, C.C.; Malanchi, I. Neutrophils in cancer: Heterogeneous and multifaceted. Nat. Rev. Immunol. 2022, 22, 173–187.
- Que, H.; Fu, Q.; Lan, T.; Tian, X.; Wei, X. Tumor-associated neutrophils and neutrophil-targeted cancer therapies. Biochim. Biophys. Acta Rev. Cancer 2022, 1877, 188762.
- Saccheri, F.; Pozzi, C.; Avogadri, F.; Barozzi, S.; Faretta, M.; Fusi, P.; Rescigno, M. Bacteria-induced gap junctions in tumors favor antigen cross-presentation and antitumor immunity. Sci. Transl. Med. 2010, 2, 44ra57.
- Chang, W.W.; Lai, C.H.; Chen, M.C.; Liu, C.F.; Kuan, Y.D.; Lin, S.T.; Lee, C.H. Salmonella enhance chemosensitivity in tumor through connexin 43 upregulation. Int. J. Cancer 2013, 133, 1926–1935.
- Chen, J.; Qiao, Y.; Tang, B.; Chen, G.; Liu, X.; Yang, B.; Wei, J.; Zhang, X.; Cheng, X.; Du, P.; et al. Modulation of Salmonella Tumor-Colonization and Intratumoral Anti-angiogenesis by Triptolide and Its Mechanism. Theranostics 2017, 7, 2250–2260.
- Johannessen, M.; Askarian, F.; Sangvik, M.; Sollid, J.E. Bacterial interference with canonical NFkappaB signalling. Microbiology 2013, 159, 2001–2013.
- Na, H.S.; Kim, H.J.; Lee, H.C.; Hong, Y.; Rhee, J.H.; Choy, H.E. Immune response induced by Salmonella typhimurium defective in ppGpp synthesis. Vaccine 2006, 24, 2027–2034.
- Al-Saafeen, B.H.; Fernandez-Cabezudo, M.J.; Al-Ramadi, B.K. Integration of Salmonella into Combination Cancer Therapy. Cancers 2021, 13, 3228.
- Pan, Y.; Yu, Y.; Wang, X.; Zhang, T. Tumor-Associated Macrophages in Tumor Immunity. Front. Immunol. 2020, 11, 583084.
- Zheng, J.H.; Nguyen, V.H.; Jiang, S.N.; Park, S.H.; Tan, W.; Hong, S.H.; Shin, M.G.; Chung, I.J.; Hong, Y.; Bom, H.S.; et al. Two-step enhanced cancer immunotherapy with engineered Salmonella typhimurium secreting heterologous flagellin. Sci. Transl. Med. 2017, 9, eaak9537.
- Xu, H.; Piao, L.; Wu, Y.; Liu, X. IFN-gamma enhances the antitumor activity of attenuated salmonella-mediated cancer immunotherapy by increasing M1 macrophage and CD4 and CD8 T cell counts and decreasing neutrophil counts. Front. Bioeng. Biotechnol. 2022, 10, 996055.
- Ohue, Y.; Nishikawa, H. Regulatory T (Treg) cells in cancer: Can Treg cells be a new therapeutic target? Cancer Sci. 2019, 110, 2080–2089.
- Gabrilovich, D.I. Myeloid-Derived Suppressor Cells. Cancer Immunol. Res. 2017, 5, 3–8.
- Vendrell, A.; Gravisaco, M.J.; Pasetti, M.F.; Croci, M.; Colombo, L.; Rodriguez, C.; Mongini, C.; Waldner, C.I. A novel Salmonella Typhi-based immunotherapy promotes tumor killing via an antitumor Th1-type cellular immune response and neutrophil activation in a mouse model of breast cancer. Vaccine 2011, 29, 728–736.
- Chang, S.Y.; Kim, Y.J.; Ko, H.J. Potential therapeutic anti-tumor effect of a Salmonella-based vaccine. Hum. Vaccin. Immunother. 2013, 9, 1654–1660.
- Hong, E.H.; Chang, S.Y.; Lee, B.R.; Pyun, A.R.; Kim, J.W.; Kweon, M.N.; Ko, H.J. Intratumoral injection of attenuated Salmonella vaccine can induce tumor microenvironmental shift from immune suppressive to immunogenic. Vaccine 2013, 31, 1377–1384.
- Chandra, D.; Jahangir, A.; Quispe-Tintaya, W.; Einstein, M.H.; Gravekamp, C. Myeloid-derived suppressor cells have a central role in attenuated Listeria monocytogenes-based immunotherapy against metastatic breast cancer in young and old mice. Br. J. Cancer 2013, 108, 2281–2290.
- Hernandez-Luna, M.A.; Luria-Perez, R. Cancer Immunotherapy: Priming the Host Immune Response with Live Attenuated Salmonella enterica. J. Immunol. Res. 2018, 2018, 2984247.
- Kim, S.H.; Castro, F.; Paterson, Y.; Gravekamp, C. High efficacy of a Listeria-based vaccine against metastatic breast cancer reveals a dual mode of action. Cancer Res. 2009, 69, 5860–5866.
- Lee, C.H.; Lin, S.T.; Liu, J.J.; Chang, W.W.; Hsieh, J.L.; Wang, W.K. Salmonella induce autophagy in melanoma by the downregulation of AKT/mTOR pathway. Gene Ther. 2014, 21, 309–316.
- Barak, Y.; Schreiber, F.; Thorne, S.H.; Contag, C.H.; Debeer, D.; Matin, A. Role of nitric oxide in Salmonella typhimurium-mediated cancer cell killing. BMC Cancer 2010, 10, 146.
- Kaur, S.; Kaur, S. Bacteriocins as Potential Anticancer Agents. Front. Pharmacol. 2015, 6, 272.
- Kim, B.J.; Forbes, N.S. Single-cell analysis demonstrates how nutrient deprivation creates apoptotic and quiescent cell populations in tumor cylindroids. Biotechnol. Bioeng. 2008, 101, 797–810.
- Li, Y.; Zhao, L.; Li, X.F. Hypoxia and the Tumor Microenvironment. Technol. Cancer Res. Treat. 2021, 20, 15330338211036304.
- Masoud, G.N.; Li, W. HIF-1alpha pathway: Role, regulation and intervention for cancer therapy. Acta Pharm. Sin. B 2015, 5, 378–389.
- Li, T.; Kang, G.; Wang, T.; Huang, H. Tumor angiogenesis and anti-angiogenic gene therapy for cancer. Oncol. Lett. 2018, 16, 687–702.
- Tu, D.G.; Chang, W.W.; Lin, S.T.; Kuo, C.Y.; Tsao, Y.T.; Lee, C.H. Salmonella inhibits tumor angiogenesis by downregulation of vascular endothelial growth factor. Oncotarget 2016, 7, 37513–37523.
- Mondal, S.; Adhikari, N.; Banerjee, S.; Amin, S.A.; Jha, T. Matrix metalloproteinase-9 (MMP-9) and its inhibitors in cancer: A minireview. Eur. J. Med. Chem. 2020, 194, 112260.
- Tsao, Y.T.; Kuo, C.Y.; Cheng, S.P.; Lee, C.H. Downregulations of AKT/mTOR Signaling Pathway for Salmonella-Mediated Suppression of Matrix Metalloproteinases-9 Expression in Mouse Tumor Models. Int. J. Mol. Sci. 2018, 19, 1630.
- Mollazadeh, S.; Sahebkar, A.; Hadizadeh, F.; Behravan, J.; Arabzadeh, S. Structural and functional aspects of P-glycoprotein and its inhibitors. Life Sci. 2018, 214, 118–123.
- Yang, C.J.; Chang, W.W.; Lin, S.T.; Chen, M.C.; Lee, C.H. Salmonella Overcomes Drug Resistance in Tumor through P-glycoprotein Downregulation. Int. J. Med. Sci. 2018, 15, 574–579.
This entry is offline, you can click here to edit this entry!
