Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Engineering, Biomedical
Focused ultrasound (FUS) has emerged as a promising noninvasive therapeutic modality for treating atherosclerotic arterial disease. High-intensity focused ultrasound (HIFU), a noninvasive and precise modality that generates high temperatures at specific target sites within tissues, has shown promising results in reducing plaque burden and improving vascular function. While low-intensity focused ultrasound (LIFU) operates at lower energy levels, promoting mild hyperthermia and stimulating tissue repair processes.
- atherosclerosis
- focused ultrasound
- high-intensity focused ultrasound
- low-intensity focused ultrasound
- treatment
1. Introduction
Atherosclerosis is a prevalent chronic inflammatory condition that prompts plaques to accrue within the arteries and is the leading case of cardiovascular disease (CVD) [1]. According to the World Health Organization’s (WHO) global statistics, in 2019, an estimated 17.9 million people died from atherosclerosis-related complications, accounting for 32% of all global deaths. Atherosclerosis is a significant risk factor caused by the accumulation of cholesterol and fatty deposits in the arteries [2]. High blood pressure, diabetes, obesity, smoking, and a sedentary lifestyle can all substantially increase the risk [3]. Medical imaging is an effective method to screen and diagnose atherosclerosis [4,5,6,7]. Common treatments for this condition include lifestyle changes, medication, and surgery. Taking proactive steps to mitigate these risks is essential to prevent the onset of atherosclerosis and CVD [8].
The progression of atherosclerosis cause narrowing and stiffness of arteries, thereby reducing blood flow to vital organs and tissues [9]. Depending on the size of the artery blockage, the severity of symptoms can vary from mild discomfort to debilitating distress [10]. Atherosclerosis is a complex process that includes the progressive accretion of plaques within the arteries, causing the narrowing of the vascular lumen and consequent cardiovascular anomalies. A study revealed the association between radial artery and coronary calcification in adults with angina symptoms and associated risk factors [11]. Endothelial dysfunction or disruption to the interior layer of the artery walls is the root cause of atherosclerosis, and numerous accompanying risk factors such as smoking, high blood pressure, and excessive cholesterol levels can trigger this dysfunction [3,9,12]. Atherosclerosis-related diseases, such as coronary artery disease (CAD) and cerebrovascular disease, massively induce CVD mortality [10]. The progression of atherosclerosis might elicit the arteries to narrow and become stiffer, diminishing blood flow to vital tissues and organs. Given the size and enormity of the artery blockage, the symptoms could vary from mild discomfort to debilitating distress. Plaques accumulate due to an inflammatory response initiated by collecting fatty deposits, cholesterol, and other chemicals. These plaques can rupture, permitting clots to form that entirely prevent blood flow through the artery. Inflammation contributes to the progression of the disease by boosting plaque growth and weakening the artery walls. Understanding the effect of atherosclerosis underscores the magnitude of practical, proactive efforts, early identification, and proper treatment to mitigate its detrimental consequences [13]. Due to this malfunction, low-density lipoprotein (LDL) cholesterol may invade the artery wall. LDL cholesterol undergoes alterations once it infiltrates the artery wall. It is then metabolized by immune cells, primarily macrophages [14]. These immune cells differentiate into foam cells, the hallmark of initial plaques instigating atherosclerosis. In the artery wall, foam cells and other cells, lipids, and cellular debris produce fatty streaks. The fatty streaks develop into more complex plaques. Smooth muscle cells permeate the plaque from the artery wall, developing a fibrous cap [15]. The fibrous cap comprises collagen and other proteins and environs a lipid-rich core formed of foam cells, cholesterol, and cellular debris. As the plaques develop in all dimensions, the artery wall condenses and hardens, restricting the arterial lumen and dynamic blood flow. The narrowing of arteries can be ascribed to plaque depositions within the artery wall and the inflammatory reaction it causes [16].
Risk factors, including hypertension, smoking, diabetes, and dyslipidemia, can prompt atherosclerosis [17]. These risk factors are classified as modifiable and non-modifiable (Figure 1). Hypertension is a significant risk factor for developing atherosclerosis, and its chronic form detrimentally impacts the endothelial lining of the arteries, rendering them prone to lipid deposition and inflammatory cell expansion. Hypertension upsurges the strain on the heart, leading the artery walls to thicken and stiffen. Tobacco smoke incorporates chemicals that induce endothelial dysfunction, augment inflammation, and lead to fatty plaque formation inside artery walls. Smoking also lowers high-density lipoprotein (HDL) cholesterol levels, the “good” cholesterol, which assists in normalizing the overall blood cholesterol level [18]. Hyperlipidemia can cause elevated blood glucose levels, leading to diabetes and hasten plaque formation and endothelial damage [19]. Men are more inclined than premenopausal [20] to suffer from atherosclerosis. However, the risk juxtaposes in postmenopausal women [21,22]. Notably, these risk factors often interact and worsen the other’s consequences. These factors, including obesity, hyperlipidemia, hypertension, and diabetes, can be managed and controlled by lifestyle changes, regular medical check-ups, and medications that can reduce the clinical consequences [23]. In contrast, there are also non-modifiable factors of atherosclerosis etiology, i.e., genetics, gender, chronic nonalcoholic fatty liver disease (NAFLD), habitat, and environmental factors.
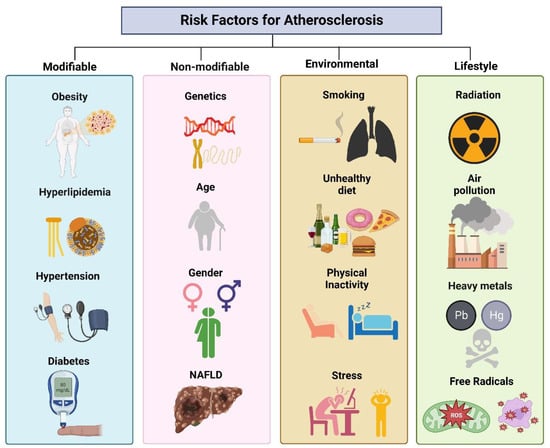
Figure 1. Association of risk factors linked with atherosclerosis.
HIFU can assist in evaluating the stages of atherosclerosis, including its potential to mitigate plaque burden, promote vascular remodeling, and reduce adverse effects compared to conventional methods. It offers targeted and precise therapeutic benefits without invasive interventions. HIFU provides high spatial resolution, enabling accurate targeting of atherosclerotic plaques without damaging surrounding healthy tissues. The ability to focus the ultrasound beam on specific locations enhances the effectiveness of the treatment and reduces the potential for collateral damage [24]. HIFU procedure is associated with minimal downtime and a faster recovery than invasive surgeries. By potentially reducing plaque burden and improving vascular function, HIFU may decrease reliance on long-term medication, mitigating the risk of contradictory effects and improving patient compliance. However, HIFU technology needs optimization for treatment protocols, addressing potential safety concerns, and conducting large-scale clinical trials to establish long-term efficacy, safety, and clinical validation. Regarding HIFU equipment, two primary components must be taken into consideration. The first component is the piezoelectric ultrasound transducer that targets the therapeutic ultrasound beam, as shown in Figure 2. Although the most utilized transducer is a concave focusing transducer with a fixed aperture and focal length, other types of transducers are available, such as phased array transducers and flat transducers [25]. The transducer’s mechanical movement determines the focal point’s position, while electronic steering offers precise control of the focal spot location. The second significant component of HIFU is the imaging modality utilized for guidance. Real-time imaging is crucial during the therapeutic procedure to ensure the safety and efficacy of treatment. Sonography and Magnetic Resonance Imaging (MRI) are two imaging modalities used for monitoring treatment [24]. Figures displaying the schematics of typical ultrasound and MRI-guided focused ultrasound systems are applied to the target through the skin for extracorporeal shock wave therapy (ESWT) and HIFU.

Figure 2. (a) FUS equipment. (b) HIFU transducers of varying sizes.
There has been an emergence of HIFU as a potential noninvasive therapeutic modality for treating atherosclerotic arterial disease in various arterial districts. The coronary, carotid, and peripheral arteries are the most accessible arterial districts for FUS application [26]. Patients will lie prone on the procedure table when undergoing FUS for the femoral artery. Real-time ultrasound or MRI imaging guides the focused ultrasound waves to the targeted location within the artery. The transducer, equipped with a cooling system, is placed on the skin over the femoral artery, allowing the ultrasound energy to target the atherosclerotic plaque precisely. This leads to localized thermal ablation and mechanical disruption of the plaque, which promotes its breakdown and eventual resorption by the body [27]. A similar procedure is followed for the carotid artery, with the patient positioned supine or slightly inclined. FUS aims to reduce plaque burden and improve blood flow to the brain, minimizing the risk of stroke or other cerebrovascular events. The cooling system ensures that the energy delivery is precisely controlled, avoiding damage to the surrounding structures [28].
2. Focused Ultrasound as an Emerging Tool for Atherosclerosis Treatment
FUS has been approved as a noninvasive treatment option for various medical conditions. This innovative modality provides highly effective therapeutic benefits and is particularly effective in treating essential tremors and ablating prostate, hepatic, breast, and uterine tumors. Using a combination of ultrasound transducers with varying shapes, frequencies, and imaging systems, FUS delivers controlled energy to the target tissue with high precision and accuracy. Patients are positioned in either a prone or supine position during the procedure, and the ultrasound waves are directed at the target site through the skin, eliminating the need for invasive incisions [40]. The potential of FUS for treating atherosclerotic arterial disease is increasingly supported by a growing body of evidence, despite pending regulatory approval. Its effectiveness has been demonstrated in various medical fields, and noninvasive delivery of focused ultrasound waves to peripheral arteries such as the femoral, carotid, and iliac arteries is feasible. This targeted approach holds great promise for reducing atherosclerotic plaque burden and improving vascular function and deserves further exploration [28].
Currently, percutaneous endovascular techniques remain the predominant approach for treating arterial pathology. However, FUS offers a noninvasive alternative that holds promise as a potential adjunct or standalone therapy for atherosclerotic arterial disease. This review thoroughly examines the current evidence and scientific literature on using noninvasive FUS for atherosclerosis treatment. Our goal is to provide a clear understanding of FUS’s potential impact on revolutionizing the management of atherosclerotic arterial disease and shaping the future of noninvasive therapeutic interventions in this field [30]. This remarkable technology has given rise to two well-recognized techniques: HIFU and LIFU. HIFU utilizes highly focused ultrasound waves to generate intense heat within specific tissues, resulting in thermal ablation and tissue dissolution [41]. It has succeeded in noninvasive tumor ablation, offering an alternative to surgical removal. HIFU has effectively treated various solid tumors, such as tumors of the prostate, liver, kidney, breast, and uterus [42]. Real-time imaging guidance is often employed during HIFU procedures to ensure accurate targeting and allow healthcare professionals to monitor the fate of the treatment [43].
HIFU has also demonstrated promise in other clinical applications, such as treating essential tremors and certain neurological disorders. LIFU, on the other hand, utilizes focused ultrasound waves of lower intensities that are not associated with thermal ablation. Despite this, LIFU offers therapeutic benefits to various clinical conditions and pathologies [18]. It has gained recognition for its ability to stimulate or influence specific organs and metabolic processes. LIFU has shown potential in various therapeutic applications, including pain management, neuromodulation, neurostimulation, and targeted drug delivery. LIFU can target specific neural centers in pain management, providing localized pain relief without systemic medications. LIFU can influence neuronal activity in neuromodulation, potentially offering noninvasive treatments for conditions like Parkinson’s disease, Alzheimer’s disease, and epilepsy [40]. LIFU can also enhance drug administration by temporarily disrupting the blood-brain barrier, enabling drugs to reach the brain.
When it comes to HIFU, precision targeting has been a fundamental challenge. However, interesting technological advancements have allowed more accurate and effective treatments [44]. The U.S. Food and Drug Administration (FDA) has approved multiple clinical applications of HIFU, including uterine fibroid ablation, prostate cancer therapy, essential tremor treatment, and relief from bone metastasis discomfort [45]. The therapeutic effects of HIFU rely on two main physical mechanisms: thermal and nonthermal. By implementing higher intensities than those used in diagnostic ultrasound imaging, HIFU achieves its curative benefits [46]. Tissue ablation and coagulative necrosis occur by converting ultrasound energy into heat when tissues absorb the energy. Cavitation, the formation and fluctuation of microbubbles, enhances the nonthermal effects of HIFU. These effects include microstreaming, jetting, bubble expansion, compression, and stable or unstable cavitation, all contributing to the disruption of the target tissue [47].
HIFU transducers are designed to converge ultrasound beams at a focal point where localized biological effects occur to ensure precise targeting. Different beam-focusing methods, such as geometric focusing, acoustic lens focusing, and electronic focusing, are employed to achieve this [48]. Geometric focusing uses the shape of the transducer’s surface to concentrate the ultrasound waves [49]. In contrast, acoustic lens focusing involves employing an acoustic lens to simulate a concave surface transducer. On the other hand, electronic focusing utilizes phased array transducers with unique excitation signals to shift the focus point electronically without physically moving the transducer [50,51,52]. It enables accurate tissue targeting with minimal impact on the surrounding area. In addition to generating and focusing ultrasound beams, HIFU systems must be able to monitor the target tissue using magnetic resonance imaging or ultrasound examination in real-time. This allows for continuous imaging supervision during the treatment [37]. A real-time dual-mode ultrasound array (DMUA) system has been developed to address this need, enabling simultaneous imaging and therapy [53].
This entry is adapted from the peer-reviewed paper 10.3390/life13081783
This entry is offline, you can click here to edit this entry!
