miRNAs are a subset of non-coding RNAs that regulate the expression of a multitude of genes post-transcriptionally and thus are potential diagnostic, prognostic, and predictive biomarkers and have also emerged as potential therapeutics. Because miRNAs are involved in the post-transcriptional regulation of their target mRNAs via repressing gene expression, defects in miRNA biogenesis pathway and miRNA expression perturb the expression of a multitude of oncogenic or tumor-suppressive genes that are involved in the pathogenesis of various cancers. As such, numerous miRNAs have been identified to be downregulated or upregulated in many cancers, functioning as either oncomes or oncosuppressor miRs. Moreover, dysregulation of miRNA biogenesis pathways can also change miRNA expression and function in cancer.
1. microRNAs
After the discovery of the first microRNA (miRNA), lin-4, in 1993 in
Caenorhabditis elegans [
79,
80], it was quickly recognized that microRNAs have been detected throughout the animal and plant kingdoms and some were shown to be highly conserved across species [
81,
82,
83], with a conserved mechanism and broad functional significance.
MiRNAs are small non-coding RNAs, with an average of 22 nucleotides in length which are highly conserved and are naturally encoded in the genomes of various species [
81,
82,
83,
84] and play key functional roles in the regulation of gene expression at the transcriptional [
85,
86,
87] and post-transcriptional [
41,
88,
89,
90,
91] levels of their target mRNAs [
41,
89] including the regulation of cell function by regulating gene expression via the modulation of the stability and translation of mRNA [
92] in a broad range of biological processes within cells and organisms, consequently affecting cell differentiation, cell proliferation, angiogenesis, and apoptosis, etc. [
48]. In addition, miRNAs exhibit tissue-specific [
67,
93] and developmental expression patterns [
75,
76,
77].
Emerging data suggest that new miRNAs are still being discovered [
94] and curreently, it is estimated that there are >2588 human mature miRNAs in human cells with time- and tissue-dependent expression patterns. Of the >2588 miRNAs, 1115 are currently annotated in miRBase V22 [
15,
16,
21,
95,
96,
97,
98]. It is estimated that these >2588 miRNAs regulate over 60% of the expression of human genes, demonstrating that they have important regulatory roles in diverse physiological and developmental processes as well as in the pathogenesis of various human diseases and disorders [
21,
22,
23,
24,
25,
26,
27,
28,
29,
30,
31,
32,
33,
34,
35,
36,
37,
38,
39,
40,
41,
42,
43,
44], including many cancers [
5,
14,
45,
46,
47,
48,
49,
50,
51,
52,
53,
54,
55,
56,
57,
58,
59,
60,
61,
62,
63,
64,
65,
66,
67,
68,
69,
70,
71,
72,
73].
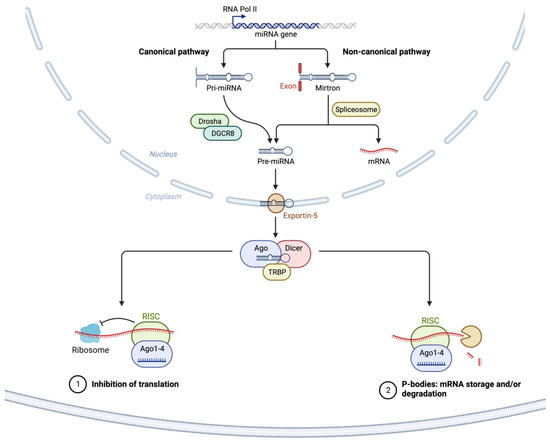
As illustrated in
Figure 1, most miRNAs are transcribed from DNA sequences into nascent primary miRNAs (pri-miRNAs) which are then initially processed by DROSHA in the nucleus to generate precursor miRNA (pre-miRNA) [
41,
99]. As many as 40% of miRNA genes may lie in the introns or even exons of other genes [
100]. Pre-miRNAs are subsequently exported from the nucleus to the cytoplasm by exportin 5 (XPO5) where they are further processed by DICER, producing small RNA duplexes with 2 nt. 3′ overhangs. These small double-stranded RNA duplexes with distinct 2 nucleotides 3′ overhangs are then loaded onto the Argonaute (AGO) protein, which retains only one strand of mature miRNA by removing the other strand [
89]. The complex formed by the AGO and miRNA associates with cofactors such as GW182 (i.e., TNRC6A), and forms the effector complex, the RNA-induced silencing complex (RISC) [
91]. The miRNA-RISC complex is responsible for the degradation of mRNA transcripts and translational suppression through interaction with the complementary mRNA target sequences predominantly located within the 3′-untranslated region (3′-UTR) of mRNAs (
Figure 1) [
101,
102,
103,
104].
Figure 1. Schematic of canonical and noncanonical miRNA biogenesis, processing, and target RNA translational suppression or degradation. The canonical miRNA pathway generates pri-miRNAs from transcripts by miRNA genes encoded in intronic, exonic, or intergenic regions. The pri-miRNA transcripts are then processed by Drosha/DGCR8 into pre-miRNAs. Intronic pre-miRNAs of the noncanonical mirtron pathway are formed by splicing, debranching, and trimming short introns without the involvement of Drosha processing. The pre-miRNA transcripts produced by both the canonical and noncanonical pathways are then exported from the nucleus to the cytoplasm by Exportin 5. This is followed by subsequent Dicer cleavage within the RISC loading complex (RLC) and subsequent unwinding of the miRNA/miRNA duplex via Argonaute, and TRBP-dependent loading into the RNA-induced silencing complex (RISC). The binding of target mRNAs to miRNAs in RISC is followed by suppression of translation and/or mRNA degradation within p-bodies in the cytosol. Created with
BioRender.com (accessed on 6 July 2023). Abbreviations: AGO2: Argonaute RISC Catalytic Component 2; DICER1: Dicer 1, Ribonuclease III; EGFR: Epidermal growth factor receptor 2; DGCR8: DiGeorge Syndrome Critical Region 8; DROSHA: Drosha Ribonuclease III; mRNA: messenger RNA; miRNA: micro RNA; P-bodies: Processing bodies; pre-miRNA: precursor microRNA; pri-miRNA: primary microRNA; RNA Pol II: RNA polymerase II; TRBP: AR RNA-binding protein; XPO5: Exportin 5.
As discussed in the literature [
59], a specific miRNA may target many different mRNAs [
105]; while a particular messenger RNA may be targeted by several miRNAs, either simultaneously or in a context-dependent fashion [
106], resulting in the cooperative repression effect [
107,
108].
2. miRNA Dysregulation in Cancer
Cancer is a complex and heterogeneous disease that evolves through successive genetic and genomic changes that support tumorigenesis [
109]. Changes in the genome that affect gene function often result from genomic alterations such as chromosomal translocations, insertions or deletions, amplifications, and single-nucleotide mutations in the epigenome. These genetic and epigenetic alterations often trigger the activation of oncogenes and suppression of tumor suppressor genes [
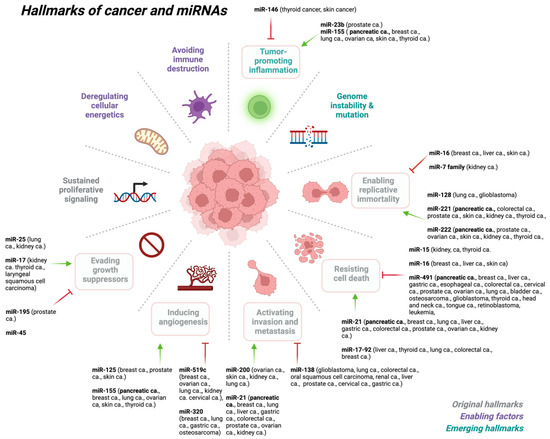
110]. To add to this picture, miRNAs also play an important role in organismal development, normal physiology, and disease state, including many hallmarks of various cancer types (
Figure 2).
Figure 2. miRNAs play key roles at various stages of cancer development. Created with
BioRender.com (accessed on 6 July 2023). Abbreviations: ca: cancer.
Although genetic, genomic, and epigenetic changes affecting genome function as well as the dysfunctions of various types of regulators are the primary molecular mechanisms responsible for the pathological processes of a multitude of human diseases, including many cancers, among which, miRNAs and their altered expression, and alterations and mutations in members of miRNA biogenesis pathways have also been recognized as additional molecular mechanisms implicated in the pathological processes of many cancers and have received great attention in recent years. Numerous studies have demonstrated the involvement of miRNAs in cancer pathogenesis by regulating the expression of their target mRNAs contributing to tumor growth, invasion, angiogenesis, and immune system evasion [
111,
112]. Furthermore, studies have shown that tumor-specific miRNA signatures can define cancer subtypes, patient survival, and treatment response [
46,
113,
114], and notably, cancer-associated miRNAs can be detected in biological fluids, allowing less-invasive monitoring [
115].
It is widely accepted that genetic deletion or amplification, epigenetic methylation of miRNA genomic loci, and alterations affecting the transcription factor-mediated regulation of primary miRNA (pri-miRNA) as well as factors involved in the miRNA biogenesis pathway frequently alter miRNA expression and function in many cancers.
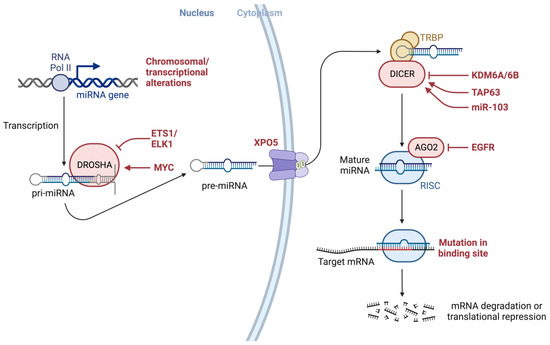
Alterations throughout miRNA biogenesis can affect the availability of target mRNA, including many mRNAs associated with cancer development (
Figure 3). As such, altered or dysregulated miRNAs or miRNA-processing machinery have been linked to many pathological processes, resulting in the failure to maintain the normal homeostatic state and eventually leading to malignant transformation, including many cancers [
5,
14,
45,
46,
47,
48,
49,
50,
51,
52,
53,
54,
55,
56,
57,
58,
59,
60,
61,
62,
63,
64,
65,
66,
67,
68,
69,
70,
71,
72,
73].
Figure 3. Alterations throughout miRNA biogenesis can affect the availability of target mRNA, including many mRNAs associated with cancer development. The figure also depicts several modulators, proteins such as EGFR, and transcription factors such as ETS1/ELK1, MYC, epigenetic regulators such as demethylating protein KDM6A, a known tumor suppressor, the tumor suppressor gene TAP63, and miRNAs such as miR-103 that may interact with modulators of miRNA-processing machinery. Both KRAS and EGFR are essential mediators of pancreatic cancer development and interact with AGO2 to perturb its function. Created with
BioRender.com (accessed on 6 July 2023). Abbreviations: AGO2: Argonaute RISC Catalytic Component 2; DICER1: Dicer 1, Ribonuclease III; DROSHA: Drosha Ribonuclease III; EGFR: Epidermal growth factor receptor 2; EGFR: Epidermal growth factor receptor, ELK1: ETS Like-1 protein Elk-1; ETS1: ETS Proto-Oncogene 1; KDM6A: Lysine (K)-specific demethylase 6A; miRNA: micro RNA; mRNA: messenger RNA; MYC: MYC Proto-Oncogene; pre-miRNA: precursor microRNA; pri-miRNA: primary microRNA; RNA Pol II: RNA polymerase II; TAP63: Tumor protein p63; XPO5: Exportin 5.
As illustrated in
Figure 3, various genetic or epigenetic alterations in key factors involving the miRNA biogenesis process can affect the availability of miRNA targets, including many targets associated with cancer development, as well as several modulators and proteins (e.g., EGFR), transcription factors (e.g., ETS1/ELK1, MYC), epigenetic regulators (e.g., demethylating protein KDM6A, a known tumor suppressor), the tumor suppressor gene TAP63, and miRNAs (e.g., miR-103) [
116] that may interact with factors involved in miRNA biogenesis machinery. It has been shown that both KRAS and EGFR are essential mediators of pancreatic cancer development and they were shown to interact with Argonaute 2 (AGO2) to perturb its function [
117].
Recent findings indicate that different types of miRNAs have been shown to regulate many of the hallmarks of cancer
Figure 2 [
71], either as a tumor suppressor (i.e., oncosuppressor miRs) or as oncogenes (i.e., oncomirs) [
118,
119,
120,
121,
122,
123,
124,
125,
126,
127,
128]. MiRNAs play a critical role in the regulation of the expression of a multitude of genes, such as the cellular responses to environmental stresses including DNA damage, hypoxia, oxidative stress, and starvation.
Supporting this, miRNAs with an oncogenic function as well as tumor-suppressing function have recently been identified in various neoplastic malignancies, and dysregulation of miRNA expression is closely associated with cancer initiation, progression, and metastasis and shown to be associated with the origin, progression, therapeutic response, and patient survival of the disease [
47,
51,
55,
62,
67,
72,
73,
129,
130].
For example, the tissue specificity of miRNAs [
67,
93], which is required for maintaining normal cell and tissue homeostasis [
46], allows them to be used as potential biomarkers in diagnosing cancer of unknown primary origin [
97,
131].
Supporting these findings, recurrent genetic and epigenetic alterations of individual miRNAs and components involved in the miRNA-processing machinery and biogenesis pathway have been identified in many cancer types, and emerging data from several functional studies provide evidence for the mechanism of action of many potential oncogenic and tumor-suppressor miRNAs [
118,
119,
120,
121,
122,
123,
124,
125,
126,
127,
128] with potential clinical applications as potential diagnostics and therapeutics.
This entry is adapted from the peer-reviewed paper 10.3390/ijms241713340