DPPH radical removal is one of the most widely applied and used methods in food and pharmaceutical applications. DPPH method gives a better response for mostly phenolic compounds and then for compounds with limited polarity. In the case of polar and phenolic compounds, adding water to the reaction medium, that is, aqueous methanol, gives better results. When testing low-polarity compounds, ethyl acetate with a radical is suitable. DPPH reaction rates depend on the steric accessibility of the radical site rather than the chemical properties of the tested antioxidant compounds. The rate at which DPPH reacts with antioxidants depends on the varying ratios of mixed SET and HAT mechanisms. The reaction mechanisms of DPPH∙ scavenging and responses are modified by many environmental and experimental factors.
1. DPPH Radicals
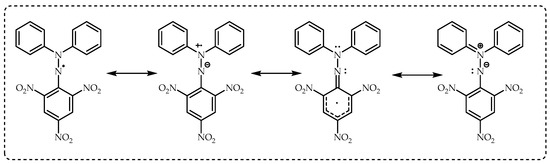
The 1,1-diphenyl-2-picrylhydrazil (DPPH) radical was discovered 100 years ago by Goldschmidt and Renn in 1922 [
78]. This method was developed by Blois [
79] using a stable free radical, DPPH, to similarly determine antioxidant activity. The chemical structures of the 1,1-diphenyl-2-picrylhydrazil radical (DPPH∙) are given in
Figure 2. This assay is based on spectrophotometric measurements of the capacity of antioxidants to scavenge DPPH radicals. Later, this test was developed in 1995 by Brand-Williams and his team and adopted by the vast majority of researchers. This antioxidant application was used very effectively by Gulcin’s research group with a slight modification [
80]. The single electron of the nitrogen atom in DPPH is reduced to the corresponding hydrazine by taking a hydrogen atom from the antioxidants. The DPPH· radical has a remarkably stable and intense color. Due to these two properties of the radical, its solution has been used intensively. This radical has been frequently used in polymer chemistry, especially in EPR spectroscopy, and in the evaluation of the antioxidant capacities of chemicals [
80,
81]. The use of this last feature in the evaluation of antioxidant capacities was first discovered by Blois in 1958 [
79]. The stability of the radicals is due to the steric crowding on the first-order divalent N atom and the “push-pull” effect exerted by the second-order diphenylamino group, an electron donor, and picryl, an electron acceptor. This effect stabilizes the canonical structure considerably. EPR measures the spin densities at the two hydrazil N atoms, which are large and essentially equal. It has been reported that the two distinct bands seen in the UV-vis spectrum of DPPH· are produced by π−π* transitions, with the unpaired electron making a major contribution to the band in the visible region [
82,
83]. When a DPPH solution is mixed with a solution of a substance capable of donating a hydrogen atom, this violet color disappears, resulting in the reduced form of the DPPH radical (DPPH-H) [
84]. The wider band is responsible for the deep violet color of the DPPH· solution. The formation of hydrazine (DPPH-H) induces the disappearance of the visible band as the color of the solution changes from violet to pale yellow as a result of radical reduction by hydrogen atom transfer from antioxidants, which are H donors. The color intensity of this reaction, known as the “DPPH test” in the literature, can be easily recorded by UV-vis spectroscopy. This method is widely used to evaluate the antioxidant capacity of pure antioxidant molecules, especially herbal extracts or phenolic compounds [
85].
Figure 2. The chemical structures of a 1,1-diphenyl-2-picrylhydrazil radical (DPPH∙).
DPPH remains a stable free radical thanks to the delocalization of the spare electron in the whole molecule (
Figure 2). In this way, the DPPH radical does not dimerize like many other free radicals. Additionally, this electron delocalization causes a dark purple color to appear in the molecule and a maximum absorption of the ethanol solution at 517 nm [
86]. Absorption is lost as the electron pairs are removed from the DPPH radical. The resulting color flare is dependent on the stoichiometry of the electron number. A concentrated solution of 0.5 mM colored alcohol also obeys Lambert-Beer’s law [
79].
While DPPH· is slightly soluble in nonpolar solvents, it dissolves quite well in different polar organic solvents. It is almost insoluble in water at room temperature. DPPH· selectively reacts with radicals and hydrogen atom donors at different reaction sites. Radicals usually attack the phenyl ring, while hydrogen donors react with the divalent nitrogen atom. The limited space around the nitrogen atom sterically inhibits the addition of bulky radicals to this region. Hydrogen atom donors can approach the nitrogen atom and release the hydrogens there with the formation of hydrazine (DPPH-H) [
81]. The researchers purposefully used a methanolic DPPH radical solution (simple to use and available as ready-made radicals) to study the antioxidant ability of food and pharmaceutical ingredients by measuring the absorbance of radicals remaining in the reaction environment [
80]. They measured residual DPPH radicals until they reached an equilibrium plateau. The simplicity of the procedure and the short reaction time made working with these radicals popular [
79]. Molecular oxygen (O
2) does not react with DPPH·. However, in the presence of light, molecular oxygen reacts slightly with DPPH·. In addition, DPPH· solutions kept in the dark can remain stable for a long time. DPPH radicals do not dimer and exist in free monomeric form in alcohol solutions [
87].
2. The Synthesis of DPPH Radicals
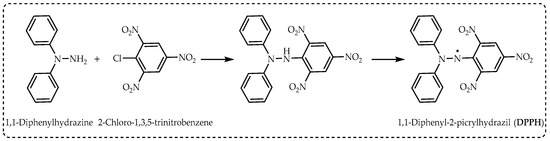
DPPH radicals are easily obtained by the oxidation of hydrazines with lead dioxide, lead tetraacetate, potassium permanganate, or silver oxide (
Figure 3). These reactions are carried out in non-polar solvents such as benzene or dichloromethane. With simple filtration, the desired radical is obtained in quantitative yield. In this way, many hydrazyl permanent or stable free radicals containing carboxyl or sulfono groups are obtained from these derivatives, generally in a single step with high yield [
3].
Figure 3. The synthesis route of 1,1-diphenyl-2-picrylhydrazil radicals (DPPH∙).
3. Interactions of DPPH· with Phenols as H-Atom Donors
Some chemicals easily react with DPPH radicals by electron transfer or by donating H atoms. Especially phenolic compounds are the most reactive and important ones that react easily with DPPH·. Hydrogen atom abstraction contains reactions that form through electron transfer followed by or preceded by proton transfer and can be formally classified as Hydrogen atom transfer reactions. The DPPH· test therefore gives an estimate of the total content of reductants present in the solution in plant extracts. The antioxidant capabilities of phenolic compounds (ArOH) are quantified by the following reaction [
1,
14]:
This radical scavenging reaction of phenolic compounds had great industrial and biological importance because it was used to reduce the oxidation rate of organic matter exposed to molecular oxygen in the air [
88,
89]. However, since peroxyl reacts very quickly with DPPH radicals, these reactions are difficult to monitor, and sophisticated devices are required. In contrast, the colored DPPH· radical is available and has much less reactivity than ROO· [
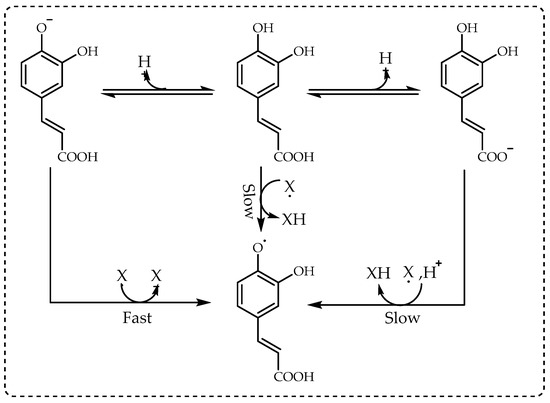
90]. As seen in
Figure 4, the best example of this situation is the electron-transfer reaction of cinnamic acids with DPPH radicals in alcoholic solutions [
91].
Figure 4. The mechanism between cinnamic acids and 1,1-diphenyl-2-picrylhydrazil (DPPH∙) radicals.
In radical scavenging studies, the antioxidant effects of phenolic compounds (Ar–OH) generally occur by two mechanisms, including hydrogen atom transfer (HAT) or single-electron transfer followed by proton transfer (SET-PT). However, in some cases, it may not be possible to separate these two mechanisms with clear boundaries [
94]. In a HAT-based assay, an antioxidant molecule can quench free radicals through H-donation, while in a SET-based method, a potential antioxidant agent exhibits antioxidant ability by transferring an electron (e-) to reduce any compound, including radicals, metals, and carbonyls [
95]. Recently, in addition to these two mechanisms, a third mechanism called the sequential proton loss electron transfer (SPLET) mechanism has been developed [
14].
The -OH group in the 7th position of flavonoids had great importance as the site of ionization and electron transfer, according to SPLET. This mechanism has been discovered recently [
96,
97]. In the first step, the reaction enthalpy corresponds to the proton affinity of the phenoxide anion (ArO
−), while in the second step, the phenoxy radical is formed by electron transfer from the phenoxide anion to ROO·. In terms of antioxidant effect, SPLET is similar to free radicals in the HAT mechanism.
4. DPPH Radical Scavenging Assays
Radical chain reactions serve as a common mechanism for lipid peroxidation. Radical scavengers increase the stability and quality of food products by ending peroxidation chain reactions. For this purpose, radical scavenger molecules interact directly with peroxide radicals and scavenge them quickly [
100]. Free radical scavenging has a known mechanism where antioxidants directly inhibit lipid peroxidation. This method is a standard, most widely used, and very fast and practical technique in antioxidant activity studies. Radical removal activity has great importance due to the hazardous effects of free radicals in foods and pharmaceutical systems. Many assays are used for the evaluation of the antioxidant activity of herbal extracts or phenolics. Different radicals and methods are used for antioxidant analyses and the determination of the final product of oxidation. ABTS
+, DPPH·, DMPD
+, or O
2− radical removal methods are the most commonly used spectrophotometric methods for this purpose. When antioxidants are added to these radicals, color removal occurs with a mechanism that reverses the formation of DPPH·, ABTS
+, and DMPD
+ cations [
1,
14].
These three radical scavenging methods are extremely fast, requiring no expensive reagents or sophisticated instruments. Preparing and analyzing a sample takes half an hour and requires very little labor. These methods, which have high sensitivity, are very easy to use. The analysis of antioxidant activity in many samples can be performed quickly and spontaneously [
14].
4.1. Evaluation of DPPH Radical Scavenging
DPPH radical scavenging ability is determined mostly in organic solvents such as methanol or ethanol by measuring the absorbance drop at 517 nm [
101]. The use of methanol is not preferred because of its toxic properties. Analyses were performed with a UV-vis spectrophotometer in 1 mL or 3 mL cuvettes. For this purpose, a stock solution of 10
−3 M DPPH radicals in ethanol or methanol was freshly prepared before analysis. To prepare the DPPH solution, 3 mL of the stock solution was diluted to 50 mL with methanol in a volumetric flask and protected from light with aluminum foil. Absorbance values were set to 1.00 ± 0.200. Then, 3 mL of DPPH working solution was transferred to the 0.5 mL extract, mixed, and left in the dark for 30 min. The purple color disappears when an antioxidant agent is present in the reaction medium. A reference sample containing 0.5 mL of solvent was similarly prepared. A newly prepared DPPH radical solution shows maximal absorption at 517 nm. All analyses were carried out in 3 replicates, and absorbance was recorded at 517 nm. The blank is the reaction mixture that does not contain test compounds [
1,
102].
4.2. Evaluation of DPPH Radical Scavenging as TEAC
Another way to evaluate the results of DPPH’s radical-removing ability is to express them as Trolox equivalent antioxidant capacity (TEAC). For this purpose, the radical-removing activity (RSA) of Trolox standard solution at different concentrations is determined. In this assay, Trolox, as a standard radical scavenger compound, is interpolated into a dose–response curve. Then, using these values (%RSA)—(Trolox; µM/L), a calibration curve is prepared. For herbal extracts or chemicals prepared at different concentrations, the TEAC (µM/L) was calculated, and Trolox equivalent antioxidant capacities were calculated using the linear regression equation obtained in the linearity range (0.01–0.05 µM/L) [
14].
4.3. The Importance of the IC50 Value in DPPH Radical Scavenging Activity
Different antioxidant concentrations are used to determine the antioxidant concentration that scavenges 50% of the initial DPPH radicals in a specific but arbitrary time interval. This concentration was also referred to as “EC
50”, short for “efficient concentration” or sometimes as “IC
50”, short for “inhibitory concentration”. Indeed, this EC
50 designation has found appropriate scientific use in drug testing under a different name “LD
50”. These terms became widely accepted as “IC
50” to indicate the practicality of antioxidant testing using DPPH radicals. The lower the IC
50 values, the higher the DPPH radical-removing ability of the antioxidants. In this context, the IC
50 value is widely used in biochemistry to compare the radical scavenging capacities of different antioxidants [
103]. The IC
50 values quantitatively describe the radical scavenging affinity. For all these reasons, the IC
50 value is one of the most practical ways to evaluate DPPH radical scavenging affinities. The radical scavenging activity (RSA) of maca extracts was calculated using the following equation:
where A
c is the absorbance at 517 nm of the control sample, and A
s is the absorbance at 517 nm that contains the test sample, including plant extracts or pure compounds. The IC
50 was calculated from the graph plotting scavenging percentage against test sample concentration (µg/mL). DPPH radicals decrease significantly upon exposure to radical remover [
104].
4.4. Scope of DPPH Radical Scavenging Applications
DPPH radical scavenging is a popular spectrophotometric method that has a wide application area and is used for determining the antioxidant capacity of beverages, pure substances, foods, and herbal extracts. This method is simple, sensitive, fast, and reproducible, making it the most convenient and common radical removal method for evaluating the antioxidant capacity of compounds and herbal extracts.
5. Limitations of the DPPH Assay
In DPPH removal activity, it is very important to convert the moles of DPPH lost by using the change in absorbance, Beer’s law, and damping ε values of 10,900–12,500. Another important point is that this method does not detect reaction rates and ignores some crucial information in the reaction curves [
160]. The reactions of DPPH and antioxidants appear to be complex when changes in absorbance are continuously monitored. Although the reaction curves were similar to ABTS
•+ removal, there were some differences when comparing the studied phenolic compounds [
85]. In DPPH radical scavenging, antioxidants can react with DPPH radicals by very fast electron transfer and slow hydrogen atom transfer. Although electron transfer is quite fast, it is slower than ABTS
•+’s reactions with antioxidants due to the difficult accessibility of phenolic compounds to the radical site of the DPPH molecule. This barrier of access inhibits all reactions, particularly the hydrogen transfer, which is necessary for the formation of a hydrogen-bonded complex between the α-C—H radical and the N lone pair required [
161,
162]. Complex molecules get in the way of each other more easily and block access to DPPH radicals at low concentrations and strongly block the reaction at high concentrations. Additionally, methyl alcohol, which is generally used as a solvent for the DPPH method, strongly binds H atoms and inhibits HAT processes [
163,
164,
165]. However, when water is added to the reaction, it disrupts the bonding and facilitates hydrogen atom transfer. Any test compound with H-atom transfer capability will increase the reaction rate. In the same manner, electron transfer is pH-dependent, with speed increasing with pH and ionization degree, while HAT is pH-independent. The dominant mechanism of a test compound can be evaluated by reacting it with DPPH radicals in methanol and 50% methanol, where the water phase is buffered to a pH range from acidic to alkaline [
85,
166,
167].
This entry is adapted from the peer-reviewed paper 10.3390/pr11082248