Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Atrial fibrillation (AF) is the most common arrhythmia worldwide. It is estimated to affect 2–4% of the adult population, and its prevalence increases with advancing age. Given the aging population, AF is expected to become even more prevalent in the coming years. Ischemic stroke and systemic embolism are the most significant risks associated with AF, leading to increased morbidity and mortality. Therefore, thromboembolism prevention is the cornerstone of managing AF.
- atrial fibrillation
- left atrial appendage occlusion
- oral anticoagulation
- stroke
1. Clinical Outcomes
Surgical eft atrial appendage occlusion (LAAO) can be performed either as a stand-alone procedure or, more commonly, as a concomitant procedure during a cardiac operation for other indications, such as valve surgery or coronary bypass grafting (CABG). The former is currently uncommon due to the increased availability of less invasive percutaneous techniques. In contrast, concomitant LAAO is carried out more frequently, and it can be categorized into two different clinical scenarios:
1.1. Concomitant Left Atrial Appendage Occlusion Surgery in Patients with Previous Atrial Fibrillation
Since the first report in 1948 [1], LAAO has been targeted in patients with a history of AF as a concomitant procedure during other cardiac operations to decrease the risk of embolic stroke. For many years, LAA exclusion was sporadically performed using non-standardized techniques as an adjunct to mitral surgery. Substantial observational evidence has been accumulated in this regard, yielding diverse results but predominantly indicating a positive impact of concomitant LAAO in preventing ischemic stroke following cardiac surgery [2]. However, the evidence was primarily based on case reports or small series with highly variable outcomes. Left atrial appendage occlusion study (LAAOS) trials have demonstrated the effectiveness and safety of LAAO in patients undergoing cardiac surgery, leading to a paradigm shift and impacting clinical practice [3][4][5]. Table 1 provides a summary of their characteristics and results.
Table 1. Characteristics and results of randomized surgical trials.
| Trial | Design | Results |
|---|---|---|
| LAAOS I (2005) |
Surgical LAAO (n = 52; suture or stapler) versus standard therapy (n = 25) |
Complete occlusion LAA: Suture 45% versus Stapler 72%; p-value = 0.14 Rate of complete occlusion LAA: initially 43% versus 87% after 4 cases (p-value = 0.0001) Perioperative thromboembolic events: Surgical LAAO 3.8% versus Control group 0%; (p-value = 1) No additional strokes at follow-up (13 ± 7 months) |
| LAAOS II (2013) |
Surgical LAAO (n = 26; ‘cut-and-sew’ technique) versus No LAAO and OAC (n = 25) |
Efficacy endpoint (1y): Compound of death, MI, stroke, SE, or major bleeding: Surgical LAAO 15.4% versus Control group 20%; (p-value = 0.61) |
| LAAOS III (2020) |
Surgical LAAO and ACO (n = 2379; suture, stapler, or LAAO device) versus no LAAO and OAC (n = 2391) |
Stroke or SE (3.8 years): Surgical LAAO 4.8% versus Control group 7%; (p-value 0.001) No differences in all-cause mortality, rehospitalization for heart failure, and myocardial infarction (3.8 years) |
| ATLAS (2022) |
LAA exclusion (n = 376; AtriClip®) versus OAC (n = 186) | Success rate (no flow nor residual stump >10 mm) of 99% Perioperative AF (1y): LAA exclusion 47.3% versus Control group 38.2%; (p-value = 0.047) Thromboembolic event after POAF (1y): LAA exclusion 3.4% versus Control group 5.6%; (p-value = 0.40) Bleeding events (1y): LAA exclusion 23% versus Control group 5.4%; (p-value = 0.005) 30-day and 1-year mortality did not show differences (p = 0.35, p = 0.36) |
LAAO: left atrial appendage occlusion; LAA: left atrial appendage; OAC: oral anticoagulation; AF: atrial fibrillation; MI: myocardial infarction; FU: follow-up; SE: systemic embolism; POAF: perioperative AF; RR: risk ratio; CI: confidence interval; HR: hazard ratio.
The Left Atrial Appendage Occlusion Study I (LAAOS I) trial was the first study to assess the safety and efficacy of LAA occlusion, using sutures or a stapling device, at the time of coronary artery bypass grafting (CABG) [3]. This research showed that a high success rate (87% of complete occlusion of the LAA after cardiac surgery) could be achieved with experience (>4 cases). In the same line, it showed good safety results, with no significant increase of operative time, bleeding, or heart failure. Except for one intraoperative ischemic stroke and one perioperative TIA, no additional strokes were detected after an average of 13 ± 7 months of follow-up.
The Left Atrial Appendage Occlusion Study II (LAAOS II) trial explored the feasibility of LAAO for stroke prevention in AF patients undergoing heart surgery [4]. After performing a cross-sectional study of 1889 consecutive patients undergoing cardiac surgery, which showed a 10.8% AF rate and 5.2% AF and increased stroke risk rate, 51 patients were randomized to LAAO or standard care. No significant differences were observed in the efficacy endpoint (composite of death, myocardial infarction (MI), stroke, noncerebral systemic emboli, or major bleeding), even though stroke was less frequent in the occlusion arm (3.9%) compared to the no occlusion arm (12%). Of note, the rate of patients recruited per center was low (1.6 per center per month).
After the publication of the LAAOS II trial, a meta-analysis summarized all available data on LAAO in patients with AF undergoing cardiac surgery [6]. A total of 3653 patients (1716 patients with concomitant LAAO versus 1937 patients without LAAO during cardiac surgery) were analyzed from two randomized trials and five observational studies. The LAAO group showed a lower incidence of stroke at 30 days (0.95% versus 1.9%; OR 0.46, p-value = 0.005) and during follow-up (1.4% versus 4.1%; OR 0.48, p-value = 0.01). The LAAO group also exhibited a significantly reduced all-cause mortality (1.9% versus 5%; OR 0.38, p-value = 0.0003), with similar rates of postoperative AF and reoperation for bleeding compared to the non-LAAO group. The authors concluded that concomitant LAAO appears to be a promising strategy for reducing the stroke risk in patients with a history of AF, both in the short and long term, without a significant increase in complications. Based on this evidence, the 2017 STS guidelines for atrial fibrillation surgery recommended LAAO during concomitant cardiac operations in patients with previous AF (Class IIa, level C) [7].
The Left Atrial Appendage Occlusion Study III (LAAOS III) trial was designed to overcome the limitations of the previous studies [5]. This multicenter randomized clinical trial assessed the role of LAAO during cardiac surgery in patients with AF and increased risk of stroke (CHA2DS2-VASc score ≥ 2). This trial was superior in the primary endpoint (first occurrence of ischemic stroke or noncerebral systemic embolism after cardiac surgery) in the LAAO group with a larger difference after the first 30 days after surgery. There were no differences in the secondary and safety endpoints, such as all-cause mortality, rehospitalization for heart failure, major bleeding, and myocardial infarction. This landmark and well-powered randomized clinical trial (RCT) provided robust evidence regarding the effectiveness of surgical LAAO during cardiac surgery in patients with AF, specifically in preventing strokes and embolisms. Notably, 76.8% of participants in both groups received OAC at the 3-year follow-up, indicating that surgical LAAO offers additional protection against strokes when combined with OAC therapy. Therefore, researchers cannot conclude that LAAO during cardiac surgery should replace OAC instead of being seen as a complement. Unlike percutaneous LAAO, this trial did not support using surgical LAAO as a stand-alone alternative to OAC therapy.
1.2. Concomitant Left Atrial Appendage Occlusion Surgery in Patients without Previous Atrial Fibrillation
The existing evidence regarding the potential embolic protection of concomitant surgical LAAO in patients without a preexisting history of AF remains unclear. Yao et al. analyzed the effect of surgical LAAO on mortality and stroke in a cohort of over 75,000 patients who underwent cardiac surgery. Among them, 25,721 (33.9%) had preexisting AF, and 4374 (5.8%) underwent LAAO. The average follow-up duration was 2.1 years. In the subgroup of patients without a previous AF, concomitant surgical LAAO was not associated with a reduced risk of stroke or mortality [8]. In a study conducted by Melduni et al. from the Mayo Clinic group, the influence of concomitant LAAO during cardiac surgery on the occurrence of perioperative AF, stroke, and all-cause mortality was assessed, involving a propensity score-matched analysis of 9792 patients [9], 54% of them with no history of previous AF. LAA closure was independently associated with an increased risk of early perioperative AF (adjusted OR, 3.88; 95% CI, 2.89–5.20) but did not significantly reduce the risk of stroke (adjusted HR, 1.07; 95% CI, 0.72–1.58) or mortality (adjusted HR, 0.92; 95% confidence interval, 0.75–1.13).
The AtriClip Left Atrial Appendage Exclusion Concomitant to Structural Heart Procedures (ATLAS) study was a prospective, randomized study that examined the feasibility of LAAO in surgical patients who developed postoperative AF [10][11]. Patients without previous AF but with a high ischemic risk (CHADS2-VASc score ≥ 2) were randomly assigned to two groups: concomitant LAAO with an AtriClip device (n = 376) and no LAAO (n = 186). The success rate of the LAAO procedure (no flow nor residual stump >10 mm) was 99%. Perioperative AF developed in 47.3% of participants in the LAAO group and 38.2% in the no LAAO group (p-value = 0.047). In patients who developed perioperative AF, thromboembolic events were observed in 3.4% of LAAO patients and 5.6% of patients without LAAO (p-value = 0.40). Based on the above evidence, surgical LAAO concomitant to cardiac surgery in patients without preexisting AF should not be recommended.
2. Surgical Techniques and Devices
LAA surgical exclusion can be achieved through various methods, which can be broadly categorized into two techniques: excision techniques and occlusion techniques [12][13][14]. Excision techniques involve resecting the LAA and suturing the remaining tissue directly or by means of a stapler. On the other hand, occlusion techniques aim to isolate the LAA from circulation while leaving it in place (Figure 1). The occlusion technique can be subdivided into endocardial direct surgical suture, stapler occlusion without excision, and device-based LAA occlusion. Table 2 provides the main classification and advantages and disadvantages of each technique.
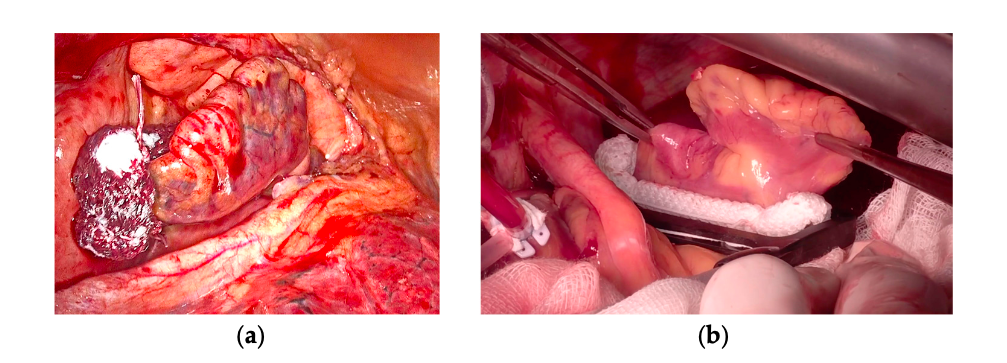
Figure 1. Left atrial appendage surgical exclusion by occlusion technique. (a) AtriClip in positioning by left thoracoscopy. (b) Measurement and implantation of AtriClip by median sternotomy.
Table 2. Characteristics of surgical LAAO techniques.
| Technique | Group | Advantage | Disadvantage |
|---|---|---|---|
| LAA resection and suture | Excision | No risk of thrombi formation (no residual stump) | Time consuming Risk of bleeding |
| Single /double layer direct suture | Occlusion | Easy to perform Cheap |
High rate of incomplete LAA exclusion |
| Stapler without stump resection | Occlusion | Easy to perform | Risk of recanalization of lumen over time |
| Stapler with stump resection | Excision | Easy to perform | Risk of bleeding |
| Occlusion devices (AtriClip) | Occlusion | Easy application Extremely high rate of effective LAA isolation Possibility of reorientation and/or reapplication Possibility of use in minimally-invasive surgery and stand-alone thoracoscopic procedures Possible role in achieving LAA electric isolation |
Cost? Risk of LAA laceration or circumflex artery injury/distortion (both very low) |
LAAO: left atrial appendage occlusion; LAA: left atrial appendage.
The initial occlusion technique for LAAO was endocardial circular purse string suture, which was later modified due to its inefficiency. They transitioned to a single- or double-layer running suture. However, despite this modification, the suture lines often remain incomplete, leading to LAA thrombosis and an elevated risk of embolism [14][15][16][17]. Katz and colleagues evaluated the efficacy of LAA endocardial ligation in patients undergoing mitral valve surgery, with no positive outcomes [18]. Incomplete exclusion was identified in 36% of the patients who underwent the procedure. Among this group, 50% also exhibited spontaneous echo contrast or thrombi in the LAA, and 22% experienced postoperative thromboembolic events. Similar results were observed in the cohort studied in 2015 by Aryana et al. [19]. These findings suggest that the technique was unsuccessful in completely excluding the left atrial appendage, leading to an increased risk of thrombotic events.
Surgical LAA closure with staplers, with or without stump excision, was then introduced as an approach to address the problem of incomplete closure. However, it was commonly observed that bleeding through the stapler line and recanalization of the lumen occurred in cases where non-excision techniques were employed [20][21].
Kanderian et al. retrospectively compared the results of three LAA exclusion techniques: surgical excision, surgical occlusion, and stapling occlusion [14]. The success rate of LAAO was modest: only 55 out of 137 (40%) LAAO were successful, the surgical excision being the most successful technique with only a 73% success rate. As for events, in a retrospective study by Lee et al., surgical excision was associated with a lower risk of stroke or TIA than all other occlusion techniques (n = 710, 0.2% versus 1.1%; p = 0.001) [22]. Among the study limitations, the low overall incidence of late neurological events and the wide variety of procedures in the “alternative techniques” group should be considered. A small pilot RCT comparing three LAA closure techniques: internal surgical ligation, surgical excision, and stapler excision confirmed the previous discouraging results, as the overall failure rate was 57%—in this case, with no significant differences between the three techniques [23].
Based on the previous results, an editorial by Marc Gillinov concluded that the “standard surgical management of the LAA is unsuccessful in the majority of cases” [24]. The shortcomings of traditional surgical techniques have led to the development of occlusion devices which effectiveness is essentially based on exerting higher and more uniform occlusion pressure than suture occlusion and stapling. The AtriClip (AtriCure, Mason, OH, USA), consisting of two polyester-covered parallel tubes with nitinol springs, is the most studied LAA occlusion device. Its application results in a necrosis line between the closure elements that effectively excludes the LAA from circulation. Other advantages include its rapid deployment and the possibility of reorientation and reapplication. Moreover, the risk of tearing the LAA or causing injury to the circumflex artery is extremely low [25]. Modified device versions have been introduced recently, allowing for minimally invasive or stand-alone total thoracoscopic procedures [26][27]. In the EXCLUDE trial, 61 patients undergoing LAA exclusion with the AtriClip were examined using TEE or CT at three months. The occlusion success rate was 98.4% [28]. Emmert et al. evaluated the long-term results of AtriClip LAA exclusion in 40 patients undergoing elective cardiac surgery. Computed tomography scans at 3, 12, 24, and 36 months showed 100% clip stability with no displacement. No thrombi, LAA perfusion, or LAA stumps were detected. Clinically, no strokes or TIAs were reported [29]. The AtriClip may also exert an anti-arrhythmogenic effect. Starck et al. showed complete electrical isolation of the LAA using AtriClip in 10 patients with AF undergoing off-pump CABG with concomitant bilateral pulmonary vein isolation [30].
Other surgical techniques include epicardial snare loops, LAA invagination and suture, and other variants described only in case reports or small series, the results of which are not generalizable on a larger scale.
3. Special Considerations in Surgical Left Atrial Appendage Management
The management of LAA can potentially address both deleterious consequences attributed to its presence in the setting of AF—namely, thrombus formation and arrhythmia persistence. It is well known that the LAA may play a role in the maintenance of advanced forms of atrial fibrillation. For this, either complete surgical excision or interruption of myocardial perfusion to the LAA (with AtriClip) will ultimately suppress the electrical contribution to AF. From a surgical standpoint, direct excision during concomitant cardiac surgery under cardiopulmonary bypass (CPB) is potentially the most effective technique for achieving embolic risk reduction and eliminating the electrical input source. Data extracted from the LAAOS III trial [5] place the surgical management of the LAA in patients with AF as a clearly advantageous, safe, and simple procedure. The inherent advantage, through surgery, of avoiding intracardiac footprints left with percutaneous occluder devices and data observed in the LAAOS III poses the question of whether more patients could benefit from minimally invasive epicardial LAA obliteration. Thrombi in the LAA constitutes a contraindication for external surgical stapling or clipping on the beating heart. Instead, such findings would favor surgical excision of the entire appendage with direct intracavitary vision and control.
Beyond the LAA excision, surgical ablation for AF has been successfully utilized for over 3 decades. However, its role and the surgical approach continue to be debated. Regardless of the AF type, a recent report demonstrated that the Cox maze procedure for stand-alone AF is safe and effective [31]. The highest one-time procedural success rate and stroke reduction were found for the Cox maze procedure with CPB compared with any catheter or off-pump surgical ablation procedure [32][33]. Thus, consideration for concomitant or stand-alone AF ablation should be given to fit patients being considered for LAA percutaneous or surgical occlusion.
Observational data strongly support the wide adoption of the most extensive AF ablation procedure (Biatrial Cox maze intervention) during concomitant cardiac surgery [34][35][36][37][38][39][40][41]. The multilevel benefits of such an intervention in terms of restoration of the sinus rhythm; control of LAA; and potentially secondary benefits (improved hemodynamics, reduced thromboembolic events, improved quality of life, restoration of left ventricular dysfunction, and potentially increased survival) make this procedure a Class I recommendation in the STS guidelines during concomitant cardiac surgery [42]. However, there is still no robust and consistent evidence arising from randomized trials on the utility of this Biatrial maze operation for many of the described hard outcomes [43][44][45][46][47].
Regarding the lifelong safety of the most used clipping or occlusion devices, very long-term data on the potential nuances of such hardware left in the intracardiac or in the pericardial space is still pending. The possibility of late erosion into the surrounding structures, late infection, or the potential to complicate cardiac interventions needs to be considered. For this, in very young patients, surgical excision and direct closure at the time of cardiac surgery seem the most appropriate and cost-effective course of action [48].
This entry is adapted from the peer-reviewed paper 10.3390/jcm12175524
References
- Madden, J.L. Resection of the Left Auricular Appendix; a Prophylaxis for Recurrent. J. Am. Med. Assoc. 1949, 140, 769–772.
- Cox, J.L.; Schuessler, R.B.; Lappas, D.G.; Boineau, J.P. An 8 1/2-Year Clinical Experience with Surgery for Atrial Fibrillation. Ann. Surg. 1996, 224, 267–275.
- Healey, J.S.; Crystal, E.; Lamy, A.; Teoh, K.; Semelhago, L.; Hohnloser, S.H.; Cybulsky, I.; Abouzahr, L.; Sawchuck, C.; Carroll, S.; et al. Left Atrial Appendage Occlusion Study (LAAOS): Results of a Randomized Controlled Pilot Study of Left Atrial Appendage Occlusion during Coronary Bypass Surgery in Patients at Risk for Stroke. Am. Heart J. 2005, 150, 288–293.
- Whitlock, R.P.; Vincent, J.; Blackall, M.H.; Hirsh, J.; Fremes, S.; Novick, R.; Devereaux, P.J.; Teoh, K.; Lamy, A.; Connolly, S.J.; et al. Left Atrial Appendage Occlusion Study II (LAAOS II). Can. J. Cardiol. 2013, 29, 1443–1447.
- Whitlock, R.P.; Belley-Cote, E.P.; Paparella, D.; Healey, J.S.; Brady, K.; Sharma, M.; Reents, W.; Budera, P.; Baddour, A.J.; Fila, P.; et al. Left Atrial Appendage Occlusion during Cardiac Surgery to Prevent Stroke. N. Engl. J. Med. 2021, 384, 2081–2091.
- Tsai, Y.C.; Phan, K.; Munkholm-larsen, S.; Tian, D.H.; La Meir, M.; Yan, T.D. Surgical Left Atrial Appendage Occlusion during Cardiac Surgery for Patients with Atrial Fibrillation: A Meta-Analysis. Eur. J. Cardiothorac. Surg. 2015, 47, 847–854.
- Badhwar, V.; Rankin, J.S.; Damiano, R.J.; Gillinov, A.M.; Bakaeen, F.G.; Edgerton, J.R.; Philpott, J.M.; McCarthy, P.M.; Bolling, S.F.; Roberts, H.G.; et al. The Society of Thoracic Surgeons 2017 Clinical Practice Guidelines for the Surgical Treatment of Atrial Fibrillation. Ann. Thorac. Surg. 2017, 103, 329–341.
- Yao, X.; Abraham, N.S.; Caleb Alexander, G.; Crown, W.; Montori, V.M.; Sangaralingham, L.R.; Gersh, B.J.; Shah, N.D.; Noseworthy, P.A. Effect of Adherence to Oral Anticoagulants on Risk of Stroke and Major Bleeding among Patients with Atrial Fibrillation. J. Am. Heart Assoc. 2016, 5, e003074.
- Melduni, R.M.; Schaff, H.V.; Lee, H.C.; Gersh, B.J.; Noseworthy, P.A.; Bailey, K.R.; Ammash, N.M.; Cha, S.S.; Fatema, K.; Wysokinski, W.E.; et al. Impact of Left Atrial Appendage Closure during Cardiac Surgery on the Occurrence of Early Postoperative Atrial Fibrillation, Stroke, and Mortality: A Propensity Score-Matched Analysis of 10,633 Patients. Circulation 2017, 135, 366–378.
- Gerdisch, M.W.; Edward Garrett, H.; Mumtaz, M.; Grehan, J.; Castillo-Sang, M.; Miller, J.; Zorn, G.; Gall, S.; Johnkoski, J.; Ramlawi, B. B-PO03-157 Prophylactic Left Atrial Appendage Exclusion in Patients Undergoing Cardiac Surgery: Results of Prospective, Multicenter, Randomized Atlas Trial. Hear. Rhythm 2021, 18, S253.
- Gerdisch, M.W.; Garrett, H.E.; Mumtaz, M.A.; Grehan, J.F.; Castillo-Sang, M.; Miller, J.S.; Zorn, G.L.; Gall, S.A.; Johnkoski, J.A.; Ramlawi, B. Prophylactic Left Atrial Appendage Exclusion in Cardiac Surgery Patients with Elevated CHA2DS2-VASc Score: Results of the Randomized ATLAS Trial. Innovations 2022, 17, 463–470.
- Left Atrial Appendage Exclusion for Prophylactic Stroke Reduction Trial-ClinicalTrials.Gov. Available online: https://classic.clinicaltrials.gov/ct2/show/NCT05478304 (accessed on 12 July 2023).
- Chatterjee, S.; Alexander, J.C.; Pearson, P.J.; Feldman, T. Left Atrial Appendage Occlusion: Lessons Learned from Surgical and Transcatheter Experiences. Ann. Thorac. Surg. 2011, 92, 2283–2292.
- Kanderian, A.S.; Gillinov, A.M.; Pettersson, G.B.; Blackstone, E.; Klein, A.L. Success of Surgical Left Atrial Appendage Closure: Assessment by Transesophageal Echocardiography. J. Am. Coll. Cardiol. 2008, 52, 924–929.
- García-Fernández, M.Á.; Pérez-David, E.; Quiles, J.; Peralta, J.; García-Rojas, I.; Bermejo, J.; Moreno, M.; Silva, J. Role of Left Atrial Appendage Obliteration in Stroke Reduction in Patients with Mitral Valve Prosthesis: A Transesophageal Echocardiographic Study. J. Am. Coll. Cardiol. 2003, 42, 1253–1258.
- Oneglia, C.; Muneretto, C.; Rusconi, C. Transesophageal Investigation of Surgically Ligated Left Atrial Appendage. Echocardiography 2004, 21, 617–619.
- Rosenzweig, B.P.; Katz, E.; Kort, S.; Schloss, M.; Kronzon, I. Thromboembolus from a Ligated Left Atrial Appendage. J. Am. Soc. Echocardiogr. 2001, 14, 396–398.
- Katz, E.S.; Tsiamtsiouris, T.; Applebaum, R.M.; Schwartzbard, A.; Tunick, P.A.; Kronzon, I. Surgical Left Atrial Appendage Ligation Is Frequently Incomplete: A Transesophageal Echocardiographic Study. J. Am. Coll. Cardiol. 2000, 36, 468–471.
- Aryana, A.; Singh, S.M.; Singh, S.K.; Neill, P.G.O. Surgical Suture Ligation of the Left Atrial Appendage: Outcomes from a Single-Center Study. J. Innov. Card. Rhythm Manag. 2015, 6, 2065–2072.
- Gillinov, A.M.; Pettersson, G.; Cosgrove, D.M. Stapled Excision of the Left Atrial Appendage. J. Thorac. Cardiovasc. Surg. 2005, 129, 679–680.
- Romanov, A.; Pokushalov, E.; Elesin, D.; Bogachev-Prokophiev, A.; Ponomarev, D.; Losik, D.; Bayramova, S.; Strelnikov, A.; Shabanov, V.; Pidanov, O.; et al. Effect of Left Atrial Appendage Excision on Procedure Outcome in Patients with Persistent Atrial Fibrillation Undergoing Surgical Ablation. Hear. Rhythm 2016, 13, 1803–1809.
- Lee, R.; Jivan, A.; Kruse, J.; McGee, E.C.; Malaisrie, S.C.; Bernstein, R.; Lapin, B.; Passman, R.; Knight, B.P.; McCarthy, P.M. Late Neurologic Events after Surgery for Atrial Fibrillation: Rare but Relevant. Ann. Thorac. Surg. 2013, 95, 126–132.
- Lee, R.; Vassallo, P.; Kruse, J.; Malaisrie, S.C.; Rigolin, V.; Andrei, A.C.; McCarthy, P. A Randomized, Prospective Pilot Comparison of 3 Atrial Appendage Elimination Techniques: Internal Ligation, Stapled Excision, and Surgical Excision. J. Thorac. Cardiovasc. Surg. 2016, 152, 1075–1080.
- Gillinov, M. The Left Atrial Appendage: Won’t Get Fooled Again. J. Thorac. Cardiovasc. Surg. 2016, 152, 1081–1082.
- Salzberg, S.P.; Gillinov, A.M.; Anyanwu, A.; Castillo, J.; Filsoufi, F.; Adams, D.H. Surgical Left Atrial Appendage Occlusion: Evaluation of a Novel Device with Magnetic Resonance Imaging. Eur. J. Cardiothorac. Surg. 2008, 34, 766–770.
- Ramlawi, B.; Bedeir, K.; Edgerton, J.R. Totally Thoracoscopic Closure of the Left Atrial Appendage. Ann. Thorac. Surg. 2019, 107, e71–e73.
- Sunagawa, G.; Karimov, J.H.; Breitbach, M.; Robinson, N.A.; Fukamachi, K. Impact of a Refined Advanced Design for Left Atrial Appendage Exclusion. Eur. J. Cardiothorac. Surg. 2017, 52, 1098–1103.
- Ailawadi, G.; Gerdisch, M.W.; Harvey, R.L.; Hooker, R.L.; Damiano, R.J.; Salamon, T.; Mack, M.J. Exclusion of the Left Atrial Appendage with a Novel Device: Early Results of a Multicenter Trial. J. Thorac. Cardiovasc. Surg. 2011, 142, 1002–1009.e1.
- Emmert, M.Y.; Puippe, G.; Baumüller, S.; Alkadhi, H.; Landmesser, U.; Plass, A.; Bettex, D.; Scherman, J.; Grünenfelder, J.; Genoni, M.; et al. Safe, Effective and Durable Epicardial Left Atrial Appendage Clip Occlusion in Patients with Atrial Fibrillation Undergoing Cardiac Surgery: First Long-Term Results from a Prospective Device Trial. Eur. J. Cardiothorac. Surg. 2014, 45, 126–131.
- Starck, C.T.; Steffel, J.; Emmert, M.Y.; Plass, A.; Mahapatra, S.; Falk, V.; Salzberg, S.P. Epicardial Left Atrial Appendage Clip Occlusion Also Provides the Electrical Isolation of the Left Atrial Appendage. Interact. Cardiovasc. Thorac. Surg. 2012, 15, 416–418.
- MacGregor, R.M.; Bakir, N.H.; Pedamallu, H.; Sinn, L.A.; Maniar, H.S.; Melby, S.J.; Damiano, R.J. Late Results Following Stand-Alone Surgical Ablation for Atrial Fibrillation. J. Thorac. Cardiovasc. Surg. 2022, 164, 1515.
- Ad, N.; Holmes, S.D.; Friehling, T. Minimally Invasive Stand-Alone Cox Maze Procedure for Persistent and Long-Standing Persistent Atrial Fibrillation: Perioperative Safety and 5-Year Outcomes. Circ. Arrhythm. Electrophysiol. 2017, 10, e005352.
- Khiabani, A.J.; MacGregor, R.M.; Bakir, N.H.; Manghelli, J.L.; Sinn, L.A.; Maniar, H.S.; Moon, M.R.; Schuessler, R.B.; Melby, S.J.; Damiano, R.J. The Long-Term Outcomes and Durability of the Cox-Maze IV Procedure for Atrial Fibrillation. J. Thorac. Cardiovasc. Surg. 2022, 163, 629–641.e7.
- Kim, H.J.; Han, K.D.; Kim, W.K.; Cho, Y.H.; Lee, S.H.; Je, H.G. Clinical Benefits of Concomitant Surgical Ablation for Atrial Fibrillation in Patients Undergoing Mitral Valve Surgery. Hear. Rhythm 2023, 20, 3–11.
- Cox, J.L.; Schuessler, R.B.; D’Agostino, H.J.; Stone, C.M.; Chang, B.C.; Cain, M.E.; Corr, P.B.; Boineau, J.P. The Surgical Treatment of Atrial Fibrillation: III. Development of a Definitive Surgical Procedure. J. Thorac. Cardiovasc. Surg. 1991, 101, 569–583.
- Iribarne, A.; DiScipio, A.W.; McCullough, J.N.; Quinn, R.; Leavitt, B.J.; Westbrook, B.M.; Robich, M.P.; Sardella, G.L.; Klemperer, J.D.; Kramer, R.S.; et al. Surgical Atrial Fibrillation Ablation Improves Long-Term Survival: A Multicenter Analysis. Ann. Thorac. Surg. 2019, 107, 135–142.
- Albåge, A.; Sartipy, U.; Kennebäck, G.; Johansson, B.; Scherstén, H.; Jidéus, L.; Albåge, A.; Jidéus, L.; Kennebäck, G.; Källner, G.; et al. Long-Term Risk of Ischemic Stroke after the Cox-Maze III Procedure for Atrial Fibrillation. Ann. Thorac. Surg. 2017, 104, 523–529.
- Musharbash, F.N.; Schill, M.R.; Sinn, L.A.; Schuessler, R.B.; Maniar, H.S.; Moon, M.R.; Melby, S.J.; Damiano, R.J. Performance of the Cox-Maze IV Procedure Is Associated with Improved Long-Term Survival in Patients with Atrial Fibrillation Undergoing Cardiac Surgery. J. Thorac. Cardiovasc. Surg. 2018, 155, 159–170.
- Louagie, Y.; Buche, M.; Eucher, P.; Schoevaerdts, J.C.; Gerard, M.; Jamart, J.; Blommaert, D. Improved Patient Survival with Concomitant Cox Maze III Procedure Compared with Heart Surgery Alone. Ann. Thorac. Surg. 2009, 87, 440–446.
- Ad, N.; Henry, L.; Hunt, S. The Impact of Surgical Ablation in Patients with Low Ejection Fraction, Heart Failure, and Atrial Fibrillation. Eur. J. Cardiothorac. Surg. 2011, 40, 70–76.
- McCarthy, P.M.; Gerdisch, M.; Philpott, J.; Barnhart, G.R.; Waldo, A.L.; Shemin, R.; Andrei, A.C.; Gaynor, S.; Ndikintum, N.; Calkins, H. Three-Year Outcomes of the Postapproval Study of the AtriCure Bipolar Radiofrequency Ablation of Permanent Atrial Fibrillation Trial. J. Thorac. Cardiovasc. Surg. 2022, 164, 519–527.e4.
- Ad, N.; Damiano, R.J.; Badhwar, V.; Calkins, H.; La Meir, M.; Nitta, T.; Doll, N.; Holmes, S.D.; Weinstein, A.A.; Gillinov, M. Expert Consensus Guidelines: Examining Surgical Ablation for Atrial Fibrillation. J. Thorac. Cardiovasc. Surg. 2017, 153, 1330–1354.e1.
- Nashef, S.A.M.; Fynn, S.; Abu-Omar, Y.; Spyt, T.J.; Mills, C.; Everett, C.C.; Fox-Rushby, J.; Singh, J.; Dalrymple-Hay, M.; Sudarshan, C.; et al. Amaze: A Randomized Controlled Trial of Adjunct Surgery for Atrial Fibrillation. Eur. J. Cardiothorac. Surg. 2018, 54, 729–737.
- Gillinov, A.M.; Gelijns, A.C.; Parides, M.K.; DeRose, J.J.; Moskowitz, A.J.; Voisine, P.; Ailawadi, G.; Bouchard, D.; Smith, P.K.; Mack, M.J.; et al. Surgical Ablation of Atrial Fibrillation during Mitral-Valve Surgery. N. Engl. J. Med. 2015, 372, 1399–1409.
- Blomström-Lundqvist, C.; Johansson, B.; Berglin, E.; Nilsson, L.; Jensen, S.M.; Thelin, S.; Holmgren, A.; Edvardsson, N.; Källner, G.; Blomström, P. A Randomized Double-Blind Study of Epicardial Left Atrial Cryoablation for Permanent Atrial Fibrillation in Patients Undergoing Mitral Valve Surgery: The SWEDish Multicentre Atrial Fibrillation Study (SWEDMAF). Eur. Heart J. 2007, 28, 2902–2908.
- Budera, P.; Straka, Z.; Osmančík, P.; Vaněk, T.; Jelínek, Š.; Hlavička, J.; Fojt, R.; Červinka, P.; Hulman, M.; Šmíd, M.; et al. Comparison of Cardiac Surgery with Left Atrial Surgical Ablation vs. Cardiac Surgery without Atrial Ablation in Patients with Coronary and/or Valvular Heart Disease plus Atrial Fibrillation: Final Results of the PRAGUE-12 Randomized Multicentre Study. Eur. Heart J. 2012, 33, 2644–2652.
- Osmancik, P.; Budera, P.; Talavera, D.; Hlavicka, J.; Herman, D.; Holy, J.; Cervinka, P.; Smid, J.; Hanak, P.; Hatala, R.; et al. Five-Year Outcomes in Cardiac Surgery Patients with Atrial Fibrillation Undergoing Concomitant Surgical Ablation versus No Ablation. The Long-Term Follow-up of the PRAGUE-12 Study. Hear. Rhythm 2019, 16, 1334–1340.
- Wang, Z.; Wang, K.; Lu, S.; Zhang, L.; Li, M.; Ju, W.; Ni, B.; Gu, W.; Shao, Y.; Chen, M. Surgical and Percutaneous Left Atrial Appendage Intervention: Silent Cerebral Embolism Considerations. Eur. J. Cardiothorac. Surg. 2023, 63.
This entry is offline, you can click here to edit this entry!
