Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Obstructive sleep apnea-hypopnea syndrome (OSAHS) and chronic obstructive pulmonary disease (COPD) are independently linked to an increase in cardiovascular disease (CVD). Only a few studies have been published linking the association between overlap syndrome and congestive heart failure (CHF). It is becoming increasingly clear through research that there is a strong connection between OSA, COPD, and CHF.
- obstructive sleep apnea hypopnea syndrome
- chronic obstructive pulmonary disease
- congestive heart failure
- overlap syndrome
1. Introduction
In 1985, David C. Flenely first used the term “overlap syndrome” (OS) to characterize the combination of chronic obstructive pulmonary disease (COPD) and obstructive sleep apnea (OSA). The overall prevalence of COPD and OSA alone is high, and studies have revealed a higher prevalence of COPD coexisting with OSA [1][2][3]. Patients with OS often experience a significant drop in their blood oxygen levels during sleep. This nocturnal oxygen desaturation is closely associated with a higher incidence of pulmonary hypertension and more extensive changes in the structure and function of the right side of the heart compared to patients who have either obstructive sleep apnea or chronic obstructive pulmonary disease alone. Other cardiovascular complications of OS include systemic hypertension, coronary artery disease, arrhythmia, and CHF [4][5]. OS carries a greater clinical significance because it is associated with significant co-morbid conditions [2]. It is prudent that clinicians maintain a vigilant attitude towards the possibility of OS, as timely recognition and appropriate management are imperative to prevent potential complications. One of the commonly encountered complications or comorbidities in OS is CHF. Hence, discussing the interplay between these three-disease entities (OSAHS-COPD-CHF) is important as a potential “triple overlap syndrome.”
It is becoming increasingly clear through research that there is a strong connection between OSA, COPD, and CHF. While these conditions have their own distinct origins and clinical features, they often coexist due to shared risk factors such as age, obesity, smoking, and systemic inflammation. Moreover, each condition’s pathophysiological mechanisms can exacerbate the others. Thus, a vicious cycle of worsening symptoms and outcomes is created. This interplay involves many factors, including the impact of OSA on COPD exacerbations and how COPD can affect cardiac function in CHF patients. Additionally, both OSA and COPD can contribute to systemic inflammation, oxidative stress, endothelial dysfunction, and neurohormonal abnormalities, which substantially increase the risk of CHF. Furthermore, CHF itself is a risk factor for OSA.
The pathogenesis of cardiovascular outcomes, including CHF in OS, is multifactorial and characterized by a cascade of events, as discussed below. Hypoxia triggers a chain of oxidative stress, systemic inflammation, and vascular dysfunction, which is further compounded by sympathetic overactivity. The influence of modifiable risk factors such as obesity and smoking further exacerbates this process.
2. Hypoxia in Triple Overlap Syndrome (OSAHS-COPD-CHF)
Patients with COPD experience decreased oxygen levels and breathing difficulties during sleep, especially during the REM (rapid eye movement) phase, due to relaxed intercostal muscles and limited chest wall movement. In contrast, patients with OSA encounter episodes of breathing cessation and reduced breathing primarily caused by the collapse of the upper airway, reduced pressure within the chest, and the activation of the sympathetic nervous system. This leads to frequent awakenings during the night and excessive sleepiness during the day [6]. Individuals who suffer from OS may encounter heightened levels of hypoxemia and hypercapnia. This is caused by a decrease in sensitivity of the respiratory center to both hypoxemia and hypercapnia stimulation. This decrease is a result of chronic hypoxia and hypercapnia, which are brought on by COPD. Additionally, intermittent nocturnal hypoxia and sleep deprivation caused by OSA may further exacerbate these symptoms [7]. The nocturnal drops in oxygen levels with an increase in carbon dioxide levels are more significant in patients with OS than those with COPD or OSA alone [6]. It is crucial to note that individuals diagnosed with OS exhibit greater levels of daytime hypoxemia and hypercapnia compared to their COPD or OSA counterparts. Hence, when combined with nocturnal hypoxemia, daytime hypoxemia and hypercapnia can potentially amplify the risk of developing CHF [7].
3. Consequences of Hypoxia on the Cardiovascular System
When cells experience low oxygen levels (hypoxia) as in congestive heart failure, it triggers the activation of a protein called HIF-1α. This protein is involved in various cellular processes, such as controlling cell death, regulating blood vessel constriction, managing energy usage, and promoting the growth of new blood vessels (angiogenesis). In addition to HIF-1α, hypoxia also activates another protein called HIF-2α, which is responsible for triggering the production of pro-inflammatory cytokines (molecules that promote inflammation). The resulting release of IL-1, IL-6, TNF-alpha, and reactive oxygen species (ROS) creates oxidative stress within the vessel wall. This oxidative stress is the driving force behind the development of atherosclerosis, as it initiates endothelial dysfunction (damage to the inner lining of blood vessels), promotes the breakdown of fats in the blood (lipid peroxidation), and sustains the disease process through angiogenesis [8][9][10].
Research has demonstrated that individuals who have both obstructive sleep apnea (OSA) and chronic obstructive pulmonary disease (COPD) exhibit elevated sympathetic activity and decreased parasympathetic activity, as evidenced by measurements of heart rate modulation. Additionally, this could promote an increase in cardiac morbidities [11]. Furthermore, overlap patients have more arterial stiffness than patients with OSA alone (Figure 1) [12].
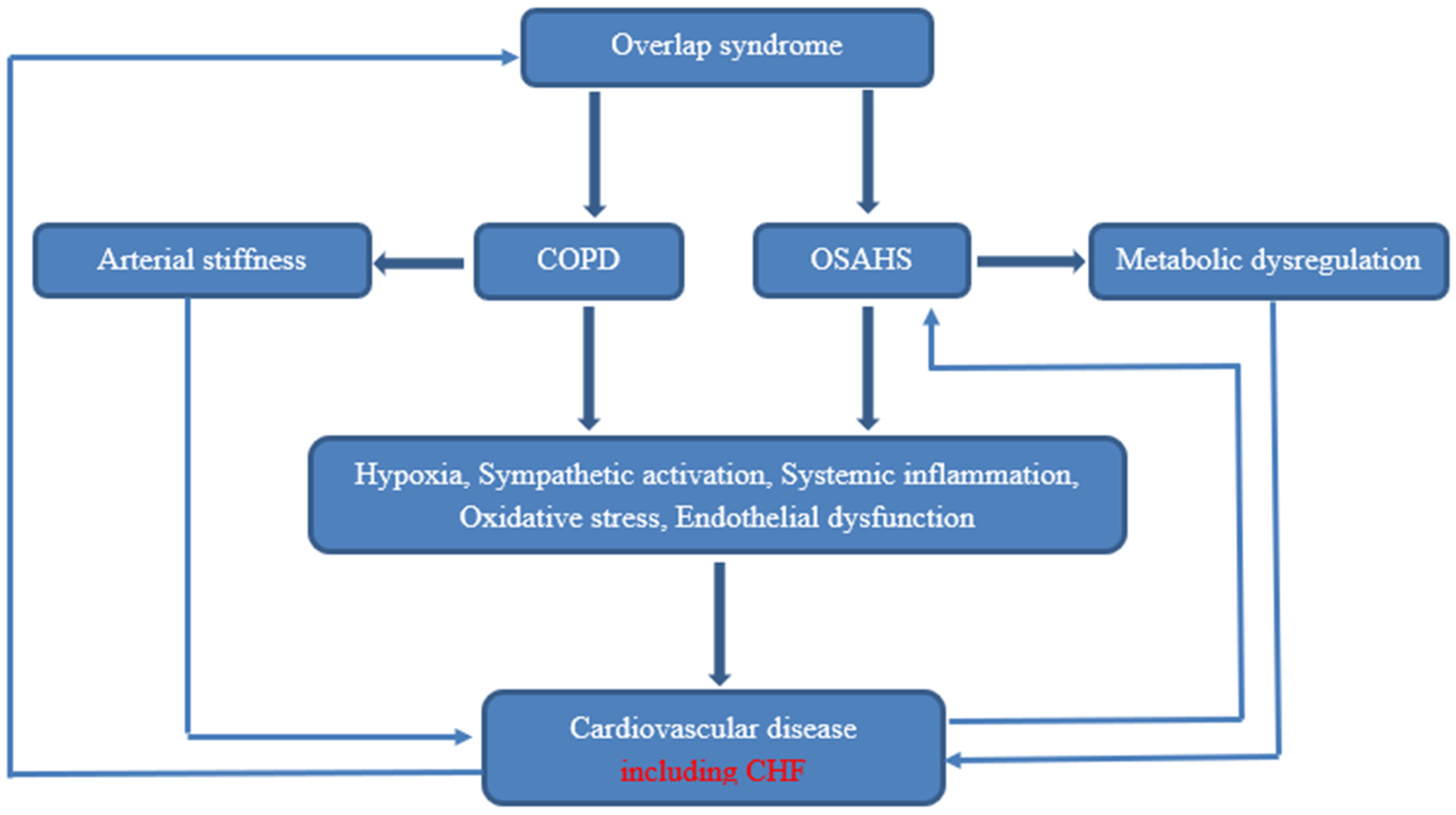
Figure 1. Pathophysiology of cardiovascular disease in OS.
4. Possible Common Contributing Factors for OSAHS, COPD, and CHF
Obesity is considered the key risk factor for OSA. Patients with COPD and OSA are more susceptible to nocturnal desaturation because of the narrowing of the upper airways caused by neck obesity. Truncal obesity may weaken the respiratory muscles and diminish the compliance of the chest wall, which could lead to ventilation-perfusion mismatches and ventilatory dysfunctions. Such disturbance in ventilatory capacity results in the retention of carbon dioxide, which can be detrimental to the cardiovascular system [13]. Cigarette smoking is a common risk factor for both COPD and OSA. Smoking can also increase oxidative stress and inflammatory mediators, increasing the pathophysiologic process that underlies CHF [14].
5. OSA in Congestive Heart Failure
Congestive heart failure (CHF) and sleep-related breathing disorders have a two-way relationship. If sleep-disordered breathing is left untreated, it can have a negative impact on the function of the left ventricle, ultimately leading to CHF [15]. According to a prospective study of 108 heart failure patients by MacDonald et al., 61% had a sleep-related breathing disorder. Of this group, 30% had OSA, and 31% had central sleep apnea [16]. Studies have shown that the prevalence of OSA is much greater in patients with CHF, ranging from 30% to 50% [16][17]. The mechanism behind Cheyne-Stokes breathing pattern caused by central sleep apnea in CHF differs from that of obstructive sleep apnea. The main pathophysiology of OSA in CHF patients is a nocturnal rostral fluid shift. During the day, fluid can build up in the lower extremities when standing up. However, when sleeping supine, this fluid mobilizes to the upper body, including the neck. This can cause swelling in the soft tissues around the pharynx and directly contribute to airway collapse and obstructive sleep apnea [18][19]. Patients with heart failure who have sleep-disordered breathing (SDB) tend to have a worse prognosis, with a higher mortality rate, in comparison to those who do not have SDB [20].
This entry is adapted from the peer-reviewed paper 10.3390/medicina59081374
References
- Varmaghani, M.; Dehghani, M.; Heidari, E.; Sharifi, F.; Moghaddam, S.S.; Farzadfar, F. Global prevalence of chronic obstructive pulmonary disease: Systematic review and meta-analysis. East. Mediterr. Heal. J. 2019, 25, 47–57.
- Zhang, P.; Chen, B.; Lou, H.; Zhu, Y.; Chen, P.; Dong, Z.; Zhu, X.; Li, T.; Lou, P. Predictors and outcomes of obstructive sleep apnea in patients with chronic obstructive pulmonary disease in China. BMC Pulm. Med. 2022, 22, 16.
- Senaratna, C.V.; Perret, J.L.; Lodge, C.J.; Lowe, A.J.; Campbell, B.E.; Matheson, M.C.; Hamilton, G.S.; Dharmage, S.C. Prevalence of obstructive sleep apnea in the general population: A systematic review. Sleep Med. Rev. 2017, 34, 70–81.
- Chaouat, A.; Naeije, R.; Weitzenblum, E. Pulmonary hypertension in COPD. Eur. Respir. J. 2008, 32, 1371–1385.
- Sharma, B.; Neilan, T.G.; Kwong, R.Y.; Mandry, D.; Owens, R.L.; McSharry, D.; Bakker, J.P.; Malhotra, A. Evaluation of Right Ventricular Remodeling Using Cardiac Magnetic Resonance Imaging in Co-Existent Chronic Obstructive Pulmonary Disease and Obstructive Sleep Apnea. COPD J. Chronic Obstr. Pulm. Dis. 2012, 10, 4–10.
- Singh, S.; Kaur, H.; Singh, S.; Khawaja, I. The Overlap Syndrome. Cureus 2018, 10, e3453.
- Tang, M.; Long, Y.; Liu, S.; Yue, X.; Shi, T. Prevalence of Cardiovascular Events and Their Risk Factors in Patients with Chronic Obstructive Pulmonary Disease and Obstructive Sleep Apnea Overlap Syndrome. Front. Cardiovasc. Med. 2021, 8, 694806.
- Higashi, Y.; Maruhashi, T.; Noma, K.; Kihara, Y. Oxidative stress and endothelial dysfunction: Clinical evidence and therapeutic implications. Trends Cardiovasc. Med. 2014, 24, 165–169.
- Sluimer, J.C.; Daemen, M.J. Novel concepts in atherogenesis: Angiogenesis and hypoxia in atherosclerosis. J. Pathol. 2009, 218, 7–29.
- Frostegård, J. Immunity, atherosclerosis and cardiovascular disease. BMC Med. 2013, 11, 117.
- Taranto-Montemurro, L.; Messineo, L.; Perger, E.; Salameh, M.; Pini, L.; Corda, L.; Ferliga, M.; Tantucci, C. Cardiac Sympathetic Hyperactivity in Patients with Chronic Obstructive Pulmonary Disease and Obstructive Sleep Apnea. COPD J. Chronic Obstr. Pulm. Dis. 2016, 13, 706–711.
- Shiina, K.; Tomiyama, H.; Takata, Y.; Yoshida, M.; Kato, K.; Nishihata, Y.; Matsumoto, C.; Odaira, M.; Saruhara, H.; Hashimura, Y.; et al. Overlap syndrome: Additive effects of COPD on the cardiovascular damages in patients with OSA. Respir. Med. 2012, 106, 1335–1341.
- Poulain, M.; Doucet, M.; Major, G.C.; Drapeau, V.; Sériès, F.; Boulet, L.-P.; Tremblay, A.; Maltais, F. The effect of obesity on chronic respiratory diseases: Pathophysiology and therapeutic strategies. Can. Med Assoc. J. 2006, 174, 1293–1299.
- Yanbaeva, D.G.; Dentener, M.A.; Creutzberg, E.C.; Wesseling, G.; Wouters, E.F.M. Systemic Effects of Smoking. Chest 2007, 131, 1557–1566.
- Javaheri, S.; Parker, T.J.; Wexler, L.; Michaels, S.E.; Stanberry, E.; Nishyama, H.; Roselle, G.A. Occult Sleep-Disordered Breathing in Stable Congestive Heart Failure. Ann. Intern. Med. 1995, 122, 487–492.
- MacDonald, M.; Fang, J.; Pittman, S.D.; White, D.P.; Malhotra, A. The current prevalence of sleep disordered breathing in congestive heart failure patients treated with beta-blockers. J. Clin. Sleep Med. 2008, 15, 38–42.
- Ferrier, K.; Campbell, A.; Yee, B.; Richards, M.; O'Meeghan, T.; Weatherall, M.; Neill, A. Sleep-Disordered Breathing Occurs Frequently in Stable Outpatients with Congestive Heart Failure. Chest 2005, 128, 2116–2122.
- Yumino, D.; Redolfi, S.; Ruttanaumpawan, P.; Su, M.C.; Smith, S.; Newton, G.E.; Mak, S.; Bradley, T.D. Nocturnal rostral fluid shift: A unifying concept for the pathogenesis of obstructive and central sleep apnea in men with heart failure. Circulation 2010, 121, 1598–1605.
- Redolfi, S.; Yumino, D.; Ruttanaumpawan, P.; Yau, B.; Su, M.-C.; Lam, J.; Bradley, T.D. Relationship between Overnight Rostral Fluid Shift and Obstructive Sleep Apnea in Nonobese Men. Am. J. Respir. Crit. Care Med. 2009, 179, 241–246.
- Lévy, P.; Naughton, M.T.; Tamisier, R.; Cowie, M.R.; Bradley, T.D. Sleep apnoea and heart failure. Eur. Respir. J. 2021, 59, 2101640.
This entry is offline, you can click here to edit this entry!
