Salmonella enterica serovars are important pathogens of humans and animals that are responsible for enormous morbidity, mortality and economic loss worldwide. Models used to study the disease pathology so far have provided valuable advancements, however, the molecular complexity of its pathogenesis remains poorly understood, particularly in humans. Therefore there remains a disconnect between what works at the bench versus at the bedside, especially in case of vaccines. The development of organoids/enteroids offers a tremendous opportunity to bridge this gap by bringing human-specific factors into the research models as well as elevate our understanding of the interactions and crosstalk between multiple cell types and the microbiota with Salmonella. Thus the use of organoids in studying Salmonella biology has the potential for improving clinical outcomes and future prophylactic and therapeutic intervention strategies.
- organoids
- enteroids
- Salmonella
- host-pathogen interactions
- model systems
- infectious diseases
- organotypic culture system
Salmonella enterica
Model Systems to Study Salmonella Biology
Organoids/Enteroids in Salmonella Biology
Organoids consist of a three-dimensional (3D) organ-like structure that is made up of organ-specific differentiated cells of multiple lineages and that recapitulates the unique organizational and functional characteristics of the corresponding organ in vivo. The 3D structures are generated in vitro from cells with a whole range of origins, such as tissue segments [3,4] and their derived adult stem cells (ASCs) [5,6], transformed cell lines [7], and pluripotent stem cells (PSCs). These models bridge the gap between simple 2D cell culture and complex in vivo experiments. Organoids have the following characteristics: 1) self-organization: individual cells arrange in vitro into a 3D structure that mimics the in vivo organ or tissue, 2) multicellularity: organoids are composed of multiple cell types typically found in the organ or tissue in equivalent proportions, 3) functionality: the organoid structure should be able to execute at least some of the organ- or tissue-specific functions, and 4) sustainability: organoids can propagate indefinitely without requiring transformation by maintaining a pool of progenitor cells. In 2012, the International Stem Cell Consortium [9] set guidelines for the nomenclature to be used to define the 3D structures generated in vitro depending on their origin and cellular composition. When referring to intestinal organotypic models, 3D structures that are composed of just epithelial cell types are generally known as “enteroids” if derived from the small intestinal epithelium, or “colonoids” if derived from colonic origin. The term “organoid” is usually reserved for 3D structures containing more than one cell lineage. However, it should be noted that these guidelines have not been uniformly adopted by the field. Many researchers commonly use “organoids” as a blanket term for 3D structures derived from ASCs, PSCs, or comprising transformed cell lines that resemble in vivo 3D architecture and physiology.
Mouse-Derived Models
Human-Derived Models
- Callejón, R.M.; Rodríguez-Naranjo, M.I.; Ubeda, C.; Hornedo-Ortega, R.; García-Parrilla, M.C.; Troncoso, A.M. Reported foodborne outbreaks due to fresh produce in the United States and European Union: Trends and causes. Foodborne Pathog. Dis. 2015, 12, 32–38. [Google Scholar] [CrossRef]
- Stanaway, J.D.; Reiner, R.C.; Blacker, B.F.; Goldberg, E.M.; Khalil, I.A.; Troeger, C.E.; Andrews, J.R.; Bhutta, Z.A.; Crump, J.A.; Im, J.; et al. The global burden of typhoid and paratyphoid fevers: A systematic analysis for the global burden of disease study 2017. Lancet Infect. Dis. 2019, 19, 369–381. [Google Scholar] [CrossRef]
- Tsolis, R.M.; Young, G.M.; Solnick, J.; Bäumler, A.J. From bench to bedside: Stealth of enteroinvasive pathogens. Nat. Rev. Genet. 2008, 6, 883–892. [Google Scholar] [CrossRef]
- Parry, C.M.; Hein, T.T.; Dougan, G.; White, N.J.; Farrar, J.J. Typhoid fever. N. Engl. J. Med. 2002, 347, 1770–1782. [Google Scholar] [CrossRef]
- Anderson, C.; Kendall, M.M. Salmonella enterica Serovar Typhimurium strategies for host adaptation. Front. Microbiol. 2017, 8, 1983. [Google Scholar] [CrossRef] [PubMed]
- Feasey, N.; Dougan, G.; Kingsley, R.A.; Heyderman, R.S.; Gordon, M.A. Invasive non-typhoidal Salmonella disease: An emerging and neglected tropical disease in Africa. Lancet 2012, 379, 2489–2499. [Google Scholar] [CrossRef]
- Dhanoa, A.; Fatt, Q.K. Non-typhoidal Salmonella bacteraemia: Epidemiology, clinical characteristics and its’ association with severe immunosuppression. Ann. Clin. Microbiol. Antimicrob. 2009, 8, 15. [Google Scholar] [CrossRef]
- Brent, A.; Oundo, J.O.; Mwangi, I.; Ochola, L.; Lowe, B.; Berkley, J.A. Salmonella bacteremia in Kenyan children. Pediatr. Infect. Dis. J. 2006, 25, 230–236. [Google Scholar] [CrossRef]
- Majowicz, S.E.; Musto, J.; Scallan, E.; Angulo, F.J.; Kirk, M.D.; O’Brien, S.J.; Jones, T.F.; Fazil, A.; Hoekstra, R.M. The global burden of nontyphoidal Salmonella gastroenteritis. Clin. Infect. Dis. 2010, 50, 882–889. [Google Scholar] [CrossRef]
- Antillon, M.; Warren, J.L.; Crawford, F.W.; Weinberger, D.M.; Kürüm, E.; Pak, G.D.; Marks, F.; Pitzer, V.E. The burden of typhoid fever in low- and middle-income countries: A meta-regression approach. PLOS Negl. Trop. Dis. 2017, 11, e0005376. [Google Scholar] [CrossRef]
- DeRoeck, D.; Jódar, L.; Clemens, J. Putting typhoid vaccination on the global health agenda. N. Engl. J. Med. 2007, 357, 1069–1071. [Google Scholar] [CrossRef] [PubMed]
- Galan, J.E.; Curtiss, R. Cloning and molecular characterization of genes whose products allow Salmonella Typhimurium to penetrate tissue culture cells. Proc. Natl. Acad. Sci. USA 1989, 86, 6383–6387. [Google Scholar] [CrossRef] [PubMed]
- Tsolis, R.M.; Adams, L.G.; Ficht, T.A.; Bäumler, A.J. Contribution of Salmonella Typhimurium virulence factors to diarrheal disease in calves. Infect. Immun. 1999, 67, 4879–4885. [Google Scholar] [CrossRef]
- Ibarra, J.A.; Steele-Mortimer, O. Salmonella—The ultimate insider. Salmonella virulence factors that modulate intracellular survival. Cell. Microbiol. 2009, 11, 1579–1586. [Google Scholar]
- Finiay, B.B.; Falkow, S. Salmonella as an intracellular parasite. Mol. Microbiol. 1989, 3, 1833–1841. [Google Scholar] [CrossRef] [PubMed]
- Richter-Dahlfors, A.; Buchan, A.; Finlay, B.B. Murine salmonellosis studied by confocal microscopy: Salmonella Typhimurium resides intracellularly inside macrophages and exerts a cytotoxic effect on phagocytes in vivo. J. Exp. Med. 1997, 186, 569–580. [Google Scholar] [CrossRef] [PubMed]
- Swart, A.L.; Hensel, M. Interactions of Salmonella enterica with dendritic cells. Virulence 2012, 3, 660–667. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Sabag-Daigle, A.; Borton, M.A.; Kop, L.F.M.; Szkoda, B.E.; Kaiser, B.L.D.; Lindemann, S.R.; Renslow, R.S.; Wei, S.; Nicora, C.D.; et al. Salmonella-mediated inflammation eliminates competitors for fructose-asparagine in the gut. Infect. Immun. 2018, 86, e00945-17. [Google Scholar] [CrossRef]
- Thiennimitr, P.; Winter, S.E.; Winter, M.G.; Xavier, M.N.; Tolstikov, V.; Huseby, U.L.; Sterzenbach, T.; Tsolis, R.M.; Roth, J.R.; Bäumler, A.J. Intestinal inflammation allows Salmonella to use ethanolamine to compete with the microbiota. Proc. Natl. Acad. Sci. USA 2011, 108, 17480–17485. [Google Scholar] [CrossRef]
- Winter, S.E.; Thiennimitr, P.; Winter, M.G.; Butler, B.P.; Huseby, U.L.; Crawford, R.W.; Russell, J.M.; Bevins, C.L.; Adams, L.G.; Tsolis, R.M.; et al. Gut inflammation provides a respiratory electron acceptor for Salmonella. Nature 2010, 467, 426–429. [Google Scholar] [CrossRef]
- Winter, S.E.; Winter, M.G.; Poon, V.; Keestra, A.M.; Sterzenbach, T.; Faber, F.; Costa, L.F.; Cassou, F.; Costa, E.A.; Alves, G.E.S.; et al. Salmonella enterica serovar Typhi conceals the invasion-associated type three secretion system from the innate immune system by gene regulation. PLoS Pathog. 2014, 10, e1004207. [Google Scholar] [CrossRef]
- Wangdi, T.; Lee, C.-Y.; Spees, A.M.; Yu, C.; Kingsbury, D.D.; Winter, S.E.; Hastey, C.J.; Wilson, R.P.; Heinrich, V.; Bäumler, A.J. The Vi capsular polysaccharide enables Salmonella enterica serovar Typhi to evade microbe-guided neutrophil chemotaxis. PLoS Pathog. 2014, 10, e1004306. [Google Scholar] [CrossRef]
- Spadoni, I.; Zagato, E.; Bertocchi, A.; Paolinelli, R.; Hot, E.; Di Sabatino, A.; Caprioli, F.; Bottiglieri, L.; Oldani, A.; Viale, G.; et al. A gut-vascular barrier controls the systemic dissemination of bacteria. Science 2015, 350, 830–834. [Google Scholar] [CrossRef]
- Hornick, R.B.; Greisman, S.E.; Woodward, T.E.; DuPont, H.L.; Dawkins, A.T.; Snyder, M.J. Typhoid fever: Pathogenesis and immunologic control. N. Engl. J. Med. 1970, 283, 686–691. [Google Scholar] [CrossRef]
- Shukla, V.; Singh, H.; Pandey, R.; Upadhyay, S.; Nath, G. Carcinoma of the Gallbladder—Is it a sequel of typhoid? Dig. Dis. Sci. 2000, 45, 900–903. [Google Scholar] [CrossRef] [PubMed]
- Caygill, C.; Hill, M.; Braddick, M.; Sharp, J. Cancer mortality in chronic typhoid and paratyphoid carriers. Lancet 1994, 343, 83–84. [Google Scholar] [CrossRef]
- Mughini-Gras, L.; Schaapveld, M.; Kramers, J.; Mooij, S.; Neefjes-Borst, E.A.; Van Pelt, W.; Neefjes, J. Increased colon cancer risk after severe Salmonella infection. PLoS ONE 2018, 13, e0189721. [Google Scholar] [CrossRef] [PubMed]
- Connerton, I.F.; Wain, J.; Hien, T.T.; Ali, T.; Parry, C.; Chinh, N.T.; Vinh, H.; Ho, V.A.; Diep, T.S.; Day, N.P.J.; et al. Epidemic typhoid in Vietnam: Molecular typing of multiple-antibiotic-resistant Salmonella enterica serotype Typhi from four outbreaks. J. Clin. Microbiol. 2000, 38, 895–897. [Google Scholar] [CrossRef]
- Molloy, A.; Nair, S.; Cooke, F.J.; Wain, J.; Farrington, M.; Lehner, P.J.; Torok, E. First report of Salmonella enterica serotype Paratyphi A azithromycin resistance leading to treatment failure. J. Clin. Microbiol. 2010, 48, 4655–4657. [Google Scholar] [CrossRef]
- Rowe, B.; Ward, L.R.; Threlfall, E.J. Multidrug-resistant Salmonella Typhi: A worldwide epidemic. Clin. Infect. Dis. 1997, 24, S106–S109. [Google Scholar] [CrossRef]
- Wang, X.; Biswas, S.; Paudyal, N.; Pan, H.; Li, X.; Fang, W.; Yue, M. Antibiotic resistance in Salmonella Typhimurium isolates recovered from the food chain through national antimicrobial resistance monitoring system between 1996 and 2016. Front. Microbiol. 2019, 10, 985. [Google Scholar] [CrossRef]
- Gopinath, S.; Lichtman, J.; Bouley, N.M.; Elias, J.E.; Monack, D.M. Role of disease-associated tolerance in infectious superspreaders. Proc. Natl. Acad. Sci. USA 2014, 111, 15780–15785. [Google Scholar] [CrossRef]
- Diard, M.; Sellin, M.E.; Dolowschiak, T.; Arnoldini, M.; Ackermann, M.; Hardt, W.-D. Antibiotic treatment selects for cooperative virulence of Salmonella Typhimurium. Curr. Boil. 2014, 24, 2000–2005. [Google Scholar] [CrossRef]
- Wotzka, S.Y.; Nguyen, B.D.; Hardt, W.-D. Salmonella Typhimurium diarrhea reveals basic principles of enteropathogen infection and disease-promoted DNA exchange. Cell Host Microbe 2017, 21, 443–454. [Google Scholar] [CrossRef]
- Acosta, C.J.; Galindo, C.; Deen, J.; Ochiai, R.; Lee, H.; Von Seidlein, L.; Carbis, R.; Clemens, J.; Ochiai, L. Vaccines against cholera, typhoid fever and shigellosis for developing countries. Expert Opin. Boil. Ther. 2004, 4, 1939–1951. [Google Scholar] [CrossRef] [PubMed]
- Ageing United Nations. Available online: https://www.un.org/en/sections/issues-depth/ageing/ (accessed on 9 February 2020).
- Featured Graphic: Many Countries’ Populations Are Aging—Population Reference Bureau. Available online: https://www.prb.org/insight/featured-graphic-many-countries-populations-are-aging/ (accessed on 9 February 2020).
- Verma, S.; Srikanth, C.V. Understanding the complexities of Salmonella-host crosstalk as revealed by in vivo model organisms. IUBMB Life 2015, 67, 482–497. [Google Scholar] [CrossRef] [PubMed]
- Criss, A.K.; Ahlgren, D.M.; Jou, T.S.; McCormick, B.A.; Casanova, J.E. The GTPase Rac1 selectively regulates Salmonella invasion at the apical plasma membrane of polarized epithelial cells. J. Cell Sci. 2001, 114, 1331–1341. [Google Scholar]
- Sun, H.; Chow, E.C.; Liu, S.; Du, Y.; Pang, K.S. The Caco-2 cell monolayer: Usefulness and limitations. Expert Opin. Drug Metab. Toxicol. 2008, 4, 395–411. [Google Scholar] [CrossRef] [PubMed]
- Sambuy, Y.; De Angelis, I.; Ranaldi, G.; Scarino, M.L.; Stammati, A.; Zucco, F. The Caco-2 cell line as a model of the intestinal barrier: Influence of cell and culture-related factors on Caco-2 cell functional characteristics. Cell Boil. Toxicol. 2005, 21, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Grossmann, J. Progress on isolation and short-term ex-vivo culture of highly purified non-apoptotic human intestinal epithelial cells (IEC). Eur. J. Cell Boil. 2003, 82, 262–270. [Google Scholar] [CrossRef]
- Aldhous, M.C.; Shmakov, A.N.; Bode, J.; Ghosh, S. Characterization of conditions for the primary culture of human small intestinal epithelial cells. Clin. Exp. Immunol. 2001, 125, 32–40. [Google Scholar] [CrossRef]
- Santos, R.L.; Tsolis, R.M.; Zhang, S.; Ficht, T.A.; Bäumler, A.J.; Adams, L.G. Salmonella-induced cell death is not required for enteritis in calves. Infect Immun. 2001, 69, 4610–4617. [Google Scholar] [CrossRef]
- Santos, R.D.L.; Zhang, S.; Tsolis, R.M.; Bäumler, A.J.; Adams, L.G. Morphologic and Molecular Characterization of Salmonella Typhimurium infection in neonatal calves. Veter Pathol. 2002, 39, 200–215. [Google Scholar] [CrossRef]
- Jepson, M.A.; Kenny, B.; Leard, A.D. Role of sipA in the early stages of Salmonella Typhimurium entry into epithelial cells. Cell Microbiol. 2001, 3, 417–426. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Santos, R.D.L.; Tsolis, R.M.; Stender, S.; Hardt, W.-D.; Bäumler, A.J.; Adams, L.G. The Salmonella enterica serotype Typhimurium effector proteins SipA, SopA, SopB, SopD, and SopE2 act in concert to induce diarrhea in calves. Infect. Immun. 2002, 70, 3843–3855. [Google Scholar] [CrossRef]
- Santos, R.D.L.; Zhang, S.; Tsolis, R.M.; Kingsley, R.A.; Adams, L.G.; Bäumler, A.J. Animal models of Salmonella infections: Enteritis versus typhoid fever. Microbes Infect. 2001, 3, 1335–1344. [Google Scholar] [CrossRef]
- Barthel, M.; Hapfelmeier, S.; Quintanilla-Martinez, L.; Kremer, M.; Rohde, M.; Hogardt, M.; Pfeffer, K.; Rüssmann, H.; Hardt, W.-D. Pretreatment of mice with streptomycin provides a Salmonella enterica serovar Typhimurium colitis model that allows analysis of both pathogen and host. Infect. Immun. 2003, 71, 2839–2858. [Google Scholar] [CrossRef] [PubMed]
- Virlogeux-Payant, I.; Waxin, H.; Ecobichon, C.; Popoff, M.Y. Role of the viaB locus in synthesis, transport and expression of Salmonella Typhi Vi antigen. Microbiology 1995, 141, 3039–3047. [Google Scholar] [CrossRef] [PubMed]
- Sabbagh, S.C.; Forest, C.G.; Lepage, C.; Leclerc, J.-M.; Daigle, F. So similar, yet so different: Uncovering distinctive features in the genomes of Salmonella enterica serovars Typhimurium and Typhi. FEMS Microbiol. Lett. 2010, 305, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Mian, M.F.; Pek, E.A.; Chenoweth, M.; Coombes, B.K.; Ashkar, A. Humanized mice for Salmonella Typhi infection: New tools for an old problem. Virulence 2011, 2, 248–252. [Google Scholar] [CrossRef] [PubMed]
- Libby, S.J.; Brehm, M.A.; Greiner, D.L.; Shultz, L.D.; McClelland, M.; Smith, K.D.; Cookson, B.T.; Karlinsey, J.E.; Kinkel, T.L.; Porwollik, S.; et al. Humanized non-obese diabetic-scid IL2rγnull mice are susceptible to lethal Salmonella Typhi infection. Proc. Natl. Acad. Sci. USA 2010, 107, 15589–15594. [Google Scholar] [CrossRef]
- Bhaduri, A.; Andrews, M.G.; Leon, W.M.; Jung, D.; Shin, D.; Allen, D.; Jung, D.; Schmunk, G.; Haeussler, M.; Salma, J.; et al. Cell stress in cortical organoids impairs molecular subtype specification. Nature 2020, 578, 142–148. [Google Scholar] [CrossRef]
- Farin, H.F.; Karthaus, W.R.; Kujala, P.; Rakhshandehroo, M.; Schwank, G.; Vries, R.G.; Kalkhoven, E.; Nieuwenhuis, E.E.; Clevers, H. Paneth cell extrusion and release of antimicrobial products is directly controlled by immune cell–derived IFN-γ. J. Exp. Med. 2014, 211, 1393–1405. [Google Scholar] [CrossRef]
- Wilson, S.S.; Tocchi, A.; Holly, M.K.; Parks, W.C.; Smith, J.G. A small intestinal organoid model of non-invasive enteric pathogen-epithelial cell interactions. Mucosal Immunol. 2014, 8, 352–361. [Google Scholar] [CrossRef]
- Wilson, C.L.; Ouellette, A.J.; Satchell, D.P.; Ayabe, T.; López-Boado, Y.S.; Stratman, J.L.; Hultgren, S.J.; Matrisian, L.M.; Parks, W.C. Regulation of intestinal-defensin activation by the metalloproteinase Matrilysin in innate host defense. Science 1999, 286, 113–117. [Google Scholar] [CrossRef] [PubMed]
- Bevins, C.L.; Salzman, N. Paneth cells, antimicrobial peptides and maintenance of intestinal homeostasis. Nat. Rev. Genet. 2011, 9, 356–368. [Google Scholar] [CrossRef] [PubMed]
- Scanu, T.; Spaapen, R.M.; Bakker, J.M.; Pratap, C.B.; Wu, L.-E.; Hofland, I.; Broeks, A.; Shukla, V.K.; Kumar, M.; Janssen, H.; et al. Salmonella manipulation of host signaling pathways provokes cellular transformation associated with gallbladder carcinoma. Cell Host Microbe 2015, 17, 763–774. [Google Scholar] [CrossRef] [PubMed]
- Kuo, S.-C.; Hu, Y.-W.; Liu, C.-J.; Lee, Y.-T.; Chen, Y.-T.; Chen, T.-L.; Chen, T.-J.; Fung, C.-P. Association between tuberculosis infections and non-pulmonary malignancies: A nationwide population-based study. Br. J. Cancer 2013, 109, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Zhan, P.; Suo, L.-J.; Qian, Q.; Shen, X.-K.; Qiu, L.-X.; Yu, L.; Song, Y. Chlamydia pneumoniae infection and lung cancer risk: A meta-analysis. Eur. J. Cancer 2011, 47, 742–747. [Google Scholar] [CrossRef] [PubMed]
- Arnheim-Dahlström, L.; Andersson, K.; Luostarinen, T.; Thoresen, S.; Ögmundsdottír, H.; Tryggvadottir, L.; Wiklund, F.; Skare, G.B.; Eklund, C.; Sjölin, K.; et al. Prospective seroepidemiologic study of uman Papillomavirus and other risk factors in cervical cancer. Cancer Epidemiol. Biomark. Prev. 2011, 20, 2541–2550. [Google Scholar] [CrossRef] [PubMed]
- Gagnaire, A.; Nadel, B.; Raoult, D.; Neefjes, J.; Gorvel, J. Collateral damage: Insights into bacterial mechanisms that predispose host cells to cancer. Nat. Rev. Genet. 2017, 15, 109–128. [Google Scholar] [CrossRef]
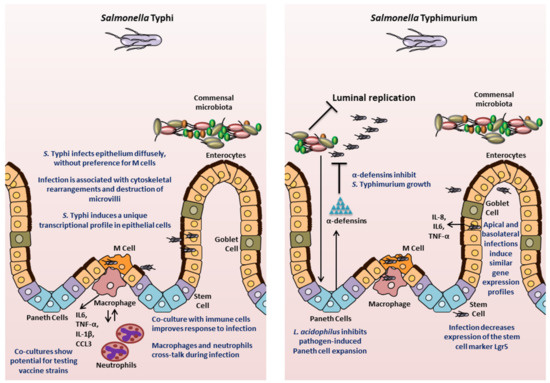
- Lu, X.; Xie, S.; Ye, L.; Zhu, L.; Yu, Q. Lactobacillus protects against S. Typhimurium –induced intestinal inflammation by determining the fate of epithelial proliferation and differentiation. Mol. Nutr. Food Res. 2020, 64, e1900655. [Google Scholar] [CrossRef]
- Li, P.; Yu, Q.; Ye, X.; Wang, Z.; Yang, Q. Lactobacillus S-layer protein inhibition of Salmonella-induced reorganization of the cytoskeleton and activation of MAPK signalling pathways in Caco-2 cells. Microbiology 2011, 157, 2639–2646. [Google Scholar] [CrossRef]
- Forbester, J.L.; Lees, E.A.; Goulding, D.; Forrest, S.; Yeung, A.; Speak, A.O.; Clare, S.; Coomber, E.L.; Mukhopadhyay, S.; Kraiczy, J.; et al. Interleukin-22 promotes phagolysosomal fusion to induce protection against Salmonella enterica Typhimurium in human epithelial cells. Proc. Natl. Acad. Sci. USA 2018, 115, 10118–10123. [Google Scholar] [CrossRef]
- Pham, T.A.N.; Clare, S.; Goulding, D.; Arasteh, J.M.; Stares, M.D.; Browne, H.P.; Keane, J.A.; Page, A.; Kumasaka, N.; Kane, L.; et al. Epithelial IL-22RA1-mediated fucosylation promotes intestinal colonization resistance to an opportunistic pathogen. Cell Host Microbe 2014, 16, 504–516. [Google Scholar] [CrossRef] [PubMed]
- Co, J.Y.; Margalef-Català, M.; Li, X.; Mah, A.T.; Kuo, C.J.; Monack, D.M.; Amieva, M. Controlling epithelial polarity: A human enteroid model for host-pathogen interactions. Cell Rep. 2019, 26, 2509–2520. [Google Scholar] [CrossRef] [PubMed]
- Salerno-Gonçalves, R.; Kayastha, D.; Fasano, A.; Levine, M.M.; Sztein, M.B. Crosstalk between leukocytes triggers differential immune responses against Salmonella enterica serovars Typhi and Paratyphi. PLOS Negl. Trop. Dis. 2019, 13, e0007650. [Google Scholar] [CrossRef] [PubMed]
- Salerno-Gonçalves, R.; Galen, J.E.; Levine, M.M.; Fasano, A.; Sztein, M.B. Manipulation of Salmonella Typhi gene expression impacts innate cell responses in the human intestinal mucosa. Front. Immunol. 2018, 9, 2543. [Google Scholar] [CrossRef] [PubMed]
- Schulte, L.N.; Schweinlin, M.; Westermann, A.J.; Janga, H.; Santos, S.C.; Appenzeller, S.; Walles, H.; Vogel, J.; Metzger, M. An advanced human intestinal coculture model reveals compartmentalized host and pathogen strategies during Salmonella infection. mBio 2020, 11, e03348-19. [Google Scholar] [CrossRef] [PubMed]
- Dougan, G.; Baker, S. Salmonella enterica serovar Typhi and the pathogenesis of typhoid fever. Annu. Rev. Microbiol. 2014, 68, 317–336. [Google Scholar] [CrossRef]
This entry is adapted from the peer-reviewed paper 10.3390/microorganisms8040504
