The prevalence of chronic diseases increases with age, especially in those with co-morbidities. The two most common denominators are the high prevalence of vitamin D deficiency and low concentrations of angiotensin-converting enzyme receptor-2 (ACE2). Whether vitamin D deficiency initiates or aggravates chronic diseases is unclear: the likelihood is both. Hypovitaminosis D negatively affects all body systems, especially the musculoskeletal and immune systems. Many chronic conditions and infections can be minimized using the right dose of vitamin D supplements administered at the right frequency (daily or once weekly) or direct sun exposure to one-third of the skin between 10:30 AM and 1:30 PM, in summer like sunlight, for 20 to 60 minutes depending on the skin tone. It is advisable to wear sunglasses and a brimmed hat to protect one’s eyes and face. Maintaining the population serum 25(OH)D concentration above 40 ng/mL (i.e., sufficiency) ensures a better immune system, minimizing symptomatic diseases and reducing infections and chronic diseases. The best clinical outcome and longevity are expected from maintaining the serum 25-hydroxyvitamin D concentrations between 50 and 80 ng/mL.
- 25(OH)D
- 1,25(OH)2D
- immune system
- SARS-CoV-2
- vitamin D deficiency
1. Introduction
Vitamin D is essential for humans. The sun-avoiding behavior (being afraid of skin cancer and darkening the skin), overusing sunscreens, living far away from the equator, and the aging process all contribute to the pandemic of hypovitaminosis. Darker-skinned people need longer sun exposure, as higher melanin filters ultraviolet-B (UVB) rays from reaching the dermis [1]. Draker shin was an evolutionary natural sunscreen to protect from excessive sun exposure in central Africa, which continues. Dark skin was a disadvantage when they migrated to the north; hence, it gradually faded. Because of lifestyle changes (e.g., predominant indoor work), despite the lighter skin color, in the absence of vitamin D supplements, people experience vitamin D deficiency and associated illnesses that intensify during winter [1].
The prevalence of chronic diseases increases with age, especially with co-morbidities. The two most common denominators are the high prevalence of vitamin D deficiency and low concentrations of angiotensin-converting enzyme receptor-2 (ACE2). Whether vitamin D deficiency initiates or aggravates chronic diseases is unclear: the likelihood is both. Hypovitaminosis D negatively affects the body, especially the musculoskeletal and immune systems. Despite lifestyle changes (e.g., predominant indoor work) over the past two centuries, despite lighter skin color, people increasingly experienced vitamin D deficiency and associated illnesses in the absence of vitamin D supplements. Unless they eat fatty fish, like salmon and mackerel, as with Scandinavians, most become vitamin D deficient, especially in winter and springtime.
Many chronic conditions and infections can be minimized using the right dose/frequency of vitamin D supplements or direct sun exposure to one-third of the skin between 10:30 AM and 1:30 PM—the shadow is shorter than height—for 30 to 60 minutes [2][3]. However, wearing sunglasses and a brimmed hat is advisable to protect one’s face and eyes. This is not feasible for many because of their geographic location, indoor work, less leisure time for sun exposure, and insufficient UVB rays reaching the surface in higher and lower latitudes. The best clinical outcome is maintaining the serum 25-hydroxyvitamin D [25(OH)Dl calcifediol] concentrations between 50 and 80 ng/mL.
Chronic hypovitaminosis D increases the risk of infections and developing complications and reduces the life span [4][5]. Further, in the north, people migrated and lived, and their skin pigment became—a survival mechanism. Fatty fish consumption provides essential fatty acids and fat-soluble vitamins, especially vitamin D. Over the past few centuries, people developed vitamin D deficiency without vitamin D supplements because of lifestyle changes—predominant indoor work—despite having lighter skin color, especially during the winter [6]. Coincidence with the troughs of severe hypovitaminosis D deficiency during winters, a high prevalence of respiratory viral diseases appears.
Parent vitamin D (D), 25-hydroxyvitamin D, and 1,25-dihydroxyvitamin D [1,25(OH)2D: calcitriol] have specific roles to play in human biology and physiology. The parent vitamin D and 25(OH)D are essential for the peripheral target cells to generate calcitriol [7]. They are vital for the synthesis of calcitriol intracellularly in proximal renal tubular cells (the hormonal form) and peripheral target cells (the non-hormonal form) for their genomic and non-genomic physiological functions [6]. The genomic effects occur when calcitriol binds to its receptor—calcitriol(vitamin D) receptor (VDR), migrates to the nucleus, binds to upstream elements, and up- or down-regulate over 1,200 genes [6]. Besides that, calcitriol has several crucial non-genomic functions [8], as discussed below.
2. Generation of Vitamin D and Transportation in the Circulation
Vitamin D is a secosteroid molecule: fully activated vitamin D, calcitriol has broad physiological functions [9][10]. These include immune modulation with anti-inflammatory and anti-oxidant actions [11], membrane stability [12], metabolic and mitochondrial respiratory functions [11], and reproductive biology. Genomic functions include the favorable transcription of over 1200 genes [13][14].
There is little vitamin D in food—e.g., D2 in sun-exposed mushrooms and D3 in fatty fish. With sufficient sun exposure, 7-dehydrocholesterol in the skin gets converted to pre-vitamin D3, which isomerizes to form vitamin D3. It binds to VDBP and diffuses via capillaries into the circulation [15]. In the small intestine, vitamin D is incorporated into VDBP and absorbed as lipoproteins/chylomicrons into the bloodstream through the thoracic duct [16]. These reach hepatocytes, where 25(OH)D is generated and released into the circulation, mostly bound to VDBP [17]. In addition to hepatocytes, 25-hydroxylase (CYP2R1 gene) is present in lesser concentrations in all peripheral target cells than in the proximal renal tubular cells [18][19][20]. Consequently, vitamin D can be converted to 25(OH)D in these cells, like immune cells [21][22] (Figure 1).
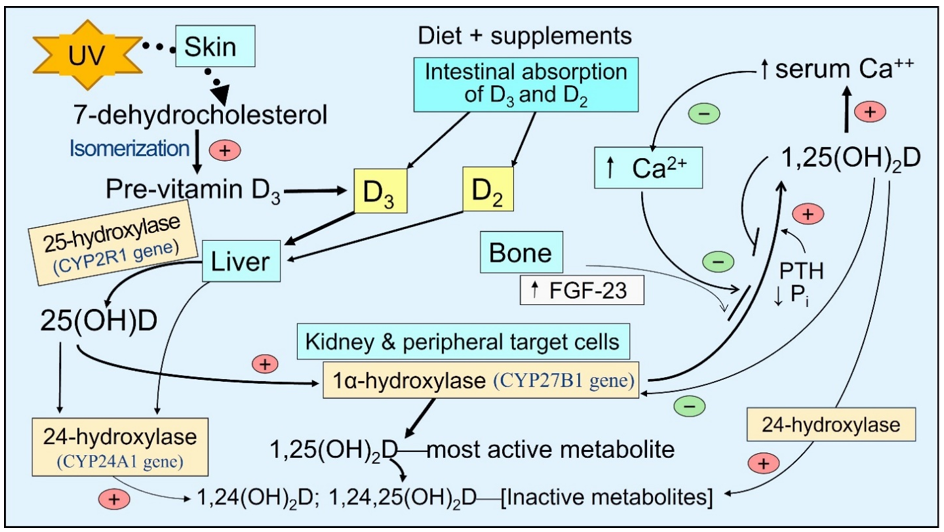
Figure 1. The basic steps involved generating and catabolizing vitamin D and 25- and 1α-hydroxylase activation steps. Synthesis of D3 in the skin—activation of vitamin D and 25(OH)D in liver and peripheral target cells by respective cytochrome, P450-hydroxylase enzymes, and 24-hydroxylase enzyme adds an OH group at 24th position of the steroid molecule, which inactivates all vitamin D products as illustrated.
The circulating half-lives of these three compounds are inversely associated with the dissociation constants [23][24]. The half-life of 25(OH)D is between two to three weeks (as it is tightly bound to VDBP), depending on the vitamin D status in the body. It is one day for vitamin D and 1,25(OH)2D for only a few hours [25]. Accordingly, the free circulating proportions are proportionately highest for 1,25(OH)2D, then D, and lowest for 25(OH)D.
After exposure to sunlight, a white-skinned person could generate up to 10,000 IU of International Units (IU) of vitamin D3 in the skin—it takes about 24 hours to materialize in circulation [26]. Ingested vitamin D3 appears in circulation between 14 and 20 h after intestinal absorption [16][26][27]. It takes about three days for the circulatory, mostly bound to VDBP 25(OH)D, to increase. Because of this short half-life, even higher doses of vitamin D (e.g., bolus doses), unless 25-hydroxylated, will be eliminated from the body in a few days [26][28]. Therefore, the best way to maintain a steady state of vitamin D and 25(OH)D in circulation is through sun exposure and/or daily supplementation [29].
3. Consequences of Vitamin D Deficiency
Since all tissues in the body need vitamin D metabolites, vitamin D deficiency negatively affects all body systems. Hypovitaminosis D increases generalized inflammation and oxidative stress, vulnerability to infections, risks for several vital diseases, and worsens all chronic conditions [30][31][32]. Besides, vitamin D has pleiotropic effects, especially on the immune, musculoskeletal, cardiovascular, pulmonary, neurological, gastrointestinal, and renal systems. Vitamin D deficiency increases the susceptibility to infections, the severity [1], complications, and deaths [33][34][35]. Figure 2 illustrates the expected consequences of chronic vitamin D deficiency.
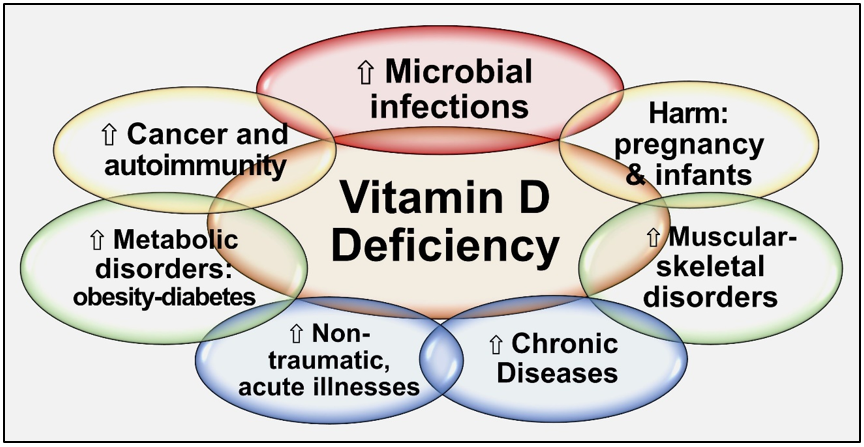
Figure 2. Summary of major adverse effects of vitamin D deficiency.
Persons with chronic kidney disease (CKD) have ineffective handling of vitamin D, 25(OH)D, and 1,25(OH)2D. This is partly due to gastrointestinal malabsorption, increased catabolism, and a significant decrease in renal 1α-hydroxylation transcribed from the CYP27B1 gene. The low circulatory D and 25(OH)D led to less formation of calcitriol, which causes hypocalcemia, secondary hyperparathyroidism), hyperphosphatemia, and elevated fibroblast growth factor-23 (FGF-23) [36]. This scenario initiates the CKD of mineral and bone disorder (CKD-MBD) [37][38]. The treatment modality of CKD-MBD has shifted from using calcitriol to providing both calcitriol and vitamin D, partly to control parathyroid hormone (PTH) and improve health and longevity [37]. This prevents secondary hyperparathyroidism and CKD-MBD [39].
Many studies have shown better survival in all types of CKD, with concomitant administration of vitamin D with calcitriol [40]. In CKD, FGF-23 increases, enhancing the expression of the CYP24A1 gene; thus, the catabolic enzyme 24-hydroxylase increases inactivation of vitamin D and its two active metabolites. This increasing serum 24-hydroxyvitamin D, 24,25-dihydroxyvitamin D, and 1,24,25-trihydroxyvitamin D to the 25(OH)D ratio in the circulation (Figure 1)—known as vitamin D catabolic (metabolic) ratio [41].
4. Muscular–Skeletal Benefits of Vitamin D
The well-known classical actions of vitamin D involve musculoskeletal functions and mineral metabolism—calcium absorption and skeletal calcification [9][42]. Skeletal functions—bone formation/ resorption and mineralization—calcitriol derived from proximal renal tubular cells (the hormonal form) work in conjunction with the parathyroid hormone (PTH) [11].
Delivering vitamin D and 25(OH)D into proximal renal tubular cells (and parathyroid cells, and storage tissues—fat and muscle) is mainly based on the active tissue transport mechanism present used by steroids and other larger molecules—megalin–cubilin endocytosis system [43]. The musculoskeletal system and PTH-driven calcitriol activities, like calcium homeostasis, are considered a part of the endocrine functions of vitamin D [44]. In contrast, the intracrine/autocrine and paracrine signaling and the biological processes of calcitriol present in peripheral target cells, like immune cells, have non-hormonal, genomic and non-genomic mechanisms. In contrast to renal tubular cells, the generation of calcitriol within immune cells by 1α-hydroxylase (CYP27B1), is dependent on the ability to diffuse enough vitamin D and/or 25(OH)D from the circulation [8][45]. This signaling mechanism is crucial for all immune cell activity.
5. Hypovitaminosis D and Recurrent Viral Respiratory Infections
During winter, respiratory tract illnesses, such as colds, influenza, and COVID-19, escalate. The countries located far north of the equator (and southern) experience higher incidences of winter-associated viral respiratory infections associated with less UVB rays in sunlight [46][47][48]. Cold and dry climate allows viruses to survive longer, and insufficient UVB rays reduce circulating D and 25(OH)D concentrations [49][50][51]. The latter significantly weakens the immune system, increasing vulnerability to infections.
Vitamin D deficiency markedly impairs overall immunity and thus increases risks of illness, like infections and metabolic disorders. This makes individuals more vulnerable to microbes [52][53], primarily intracellular bacteria like mycobacterium tuberculosis, and respiratory viral diseases [54][55][56][57][58][59], including coronaviruses [60][61][62]. Vitamin D adequacy—having blood levels greater than 30 ng/mL (older definition) [63] but preferably greater than 50 ng/mL during winter and viral epidemics—significantly reduces the risk of respiratory viral infections [4][56][64].
Children have more robust innate immune systems than older people; thus, they have better innate immunity. Consequently, unsurprisingly, they had fewer complications and deaths from SARS-CoV-2, except for those with severe hypovitaminosis D [65]. When children with severe vitamin D deficiency (i.e., serum 25(OH)D concentrations of less than 12 ng/mL) are exposed to a high viral load, they could experience severe hyperimmune reactions [66][67][68]. They are at increased risk for developing fatal immunological disorders, like Kawasaki-like disease and multi-system inflammatory syndrome [66][69].
6. Calcitriol-Mediated Intracrine/Autocrine signaling
Sufficient calcitriol synthesis within immune cells prevents inflammation, oxidative stress, infections, and autoimmune diseases [30][31]. Calcitriol suppresses the expression of inflammatory cytokines and increases the expression of anti-inflammatory cytokines and anti-oxidants [59][70]. Most chronic diseases are accompanied by chronic inflammation, which maintains the condition [30]. In addition, calcitriol enhances the production and release of antimicrobial peptides, cathelicidin, and beta-defensin via its autocrine and paracrine signaling. These antimicrobial peptides and proteins are crucial in intrinsic defense against intracellular microorganisms [31]. Intracellular signaling is also involved in adaptive immunity—the generation of neutralizing antibodies by plasma cells (Figure 1).
Cathelicidin also acts as a secondary messenger, augmenting vitamin D-mediated reduction in inflammation during infection [71]. As a non-genomic action, calcitriol stabilizes tight (gap) junctions of epithelial cells of the respiratory tract and cardiovascular system, protecting them from fluid leakage and viral dissemination into soft tissues [72][73]. Figure 3 illustrates the generation of calcitriol and the critical difference between the hormonal form and the non-hormonal form of calcitriol.
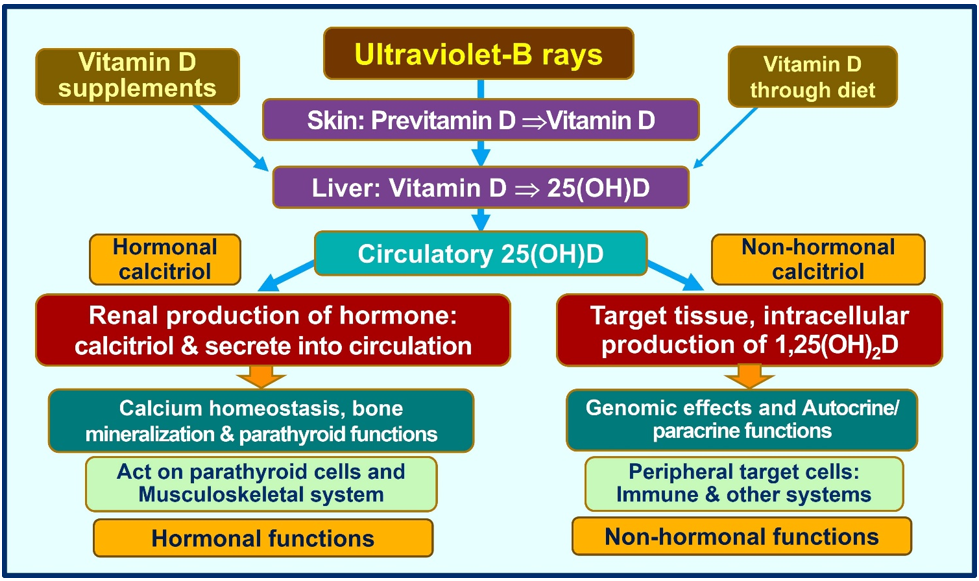
Figure 3. Humans should predominantly generate vitamin D via exposure to ultraviolet-B rays. A little vitamin D is derived from diet. Figure exemplifies the main differences between the circulatory hormonal form of calcitriol (generated via renal tubular cells) vs. the intracellularly generated calcitriol in peripheral target cells (like all immune cells).
7. Importance of Circulatory Vitamin D and 25(OH)D for Target Cell Calcitriol
Many vitamin D-related advances were made during the past decade in understanding the role of vitamin D metabolites in human biology and clinical immunology, and the physiology of calcifediol and calcitriol delineated how and when to use these agents appropriately [1][43][74]. While the musculoskeletal system functions could be maintained with smaller doses, between 800 and 1,000 IU/day, higher amounts, like 5,000 to 10,000 IU per day (or 50,000 IU once a week (once in ten days), are necessary for a non-obese 70 kg adult to maintain serum 25(OH)D concentrations above 50 ng/mL, which are needed to overcome infections [8][45].
Those who are obese, taking medications that increase vitamin D catabolism (e.g., anti-epileptic and retroviral agents), or have significant fat malabsorption require three to sixfold higher doses than those mentioned above [1][74]. Even with daily recommended amounts, vitamin D-deficient persons would take several months to increase their serum 25(OH)D above minimum therapeutic levels of 50 ng/mL [45]. With the mentioned vitamin D doses, even a person with serum 25(OH)D between 30 and 40 ng/mL would take a few weeks to raise above 50 ng/mL [8]. Therefore, such doses are insufficient to achieve the desired target serum 25(OH)D concentration in emergencies.
Chronic diseases are universally associated with low serum 25(OH)D concentrations. Examples include metabolic disorders, obesity, cancer, and infection [75][76][77][78]. Less frequent administration (i.e., intervals of less than once a month—or intermittent bolus dosing), like 300,000 every four months, does not generate the intended clinical outcomes. This is because the half-life of vitamin D is about one day, and 25(OH)D is between two to three weeks (depending on vitamin D status). Consequently, no matter the doses, the serum 25(OH)D concentration will not be maintained for over three months [79][80][81]. In addition, infrequent high-dose administration leads to unphysiological fluctuation of D metabolites, which could be harmful.
8. Vitamin D Intake Should be based on Body Weight/Fat to Achieve the Desired Serum 25(OH)D Concentration
Daily and once-a-week doses of vitamin D maintain stable circulating 25(OH)D concentrations [29]. In contrast, ingesting vitamin D for longer than monthly intervals result in significant fluctuations of circulatory 25(OH)D concentration, which is unphysiological [82][83][84]. Steady-state serum 25(OH)D concentrations, primarily depend on body weight (BW) and fat mass. The following is a simplified version of calculating the vitamin D dose needed for an individual based on BMI (body weight and fat mass) for all body weight groups [8][45].
Not obese (average wt.: BMI, <29): 70-90 IU/kg BW
Moderately obese (BMI, 30-39): 100-130 IU/kg BW
Morbid obesity (BMI, over 40): 140-180 IU/kg BW
Vitamin D supplementation and sufficient UV exposure increase circulating 25(OH)D concentration in maternal blood and breast milk [85][86]. Infants who are entirely breastfed develop vitamin D deficiency [87]. This is easily corrected with vitamin D drops given to nursing infants [88]. For each 1000 IU/d of vitamin D3-supplemented to a lactating mother, vitamin D concentration in her breast milk increased by about 80 IU/L. The recommended average dose of vitamin D3 for pregnant and lactating mothers is 6000 IU/d; this provides the infants with 400–500 IU of vitamin D per day [89].
The circulating concentration of 25(OH)D in the fetus is approximately 70% of that of the mother; thus, a diffusion of 25(OH)D occurs across the placenta [90]. However, since vitamin D concentrations are slightly below the maternal concentrations, relatively lower amounts are diffused via the placenta [29]. The same phenomenon has been reported in transferring vitamin D and 25(OH)D to breast milk [90][91]. The diffusion gradient can be increased by raising the maternal serum 25(OHD to 50 ng/mL [92].
9. Discussions
Those with co-morbidities and chronic diseases have hypovitaminosis D and, thus, are at a higher risk for developing complications from infections like SARS-CoV-2 [93]. During epidemics and pandemics, there should be an established national protocol and a mechanism for the nationwide distribution of vitamin D supplements. This is the most cost-effective way to reduce morbidity, mortality, and healthcare costs. Besides, vitamin D sufficiency would reduce the incidence and severity of chronic diseases, such as metabolic disorders (e.g., diabetes, obesity, insulin resistance), cancer, autoimmune disorders, and infections [94][95][96][97][98].
With emerging new mutant Omicron viruses (EG.5, FL.1.5.1 and BA.2.86, and Eire), the efficacy of COVID-19 vaccines and bivalent booster doses had waded and is no better than placebos [99][100]. Consequently, breakthrough SARS-CoV-2 infections occur in fully vaccinated and unvaccinated individuals [101]. It is a matter of time before the next dominant mutant manifests. However, the effectiveness of vitamin D and ivermectin has not changed [8]. Community vitamin D sufficiency is the key to protecting vulnerable populations, especially older people, ethnic minorities with darker skin color, and institutionalized persons [1][102][103][104].
A balanced diet with adequate micronutrients, such as vitamins D, B2, K2, and C, magnesium, trace minerals (zinc and selenium), resveratrol, essential fatty acids, such as omega-3, and anti-oxidants, will support a more robust immune system and good health [105][106][107][108]. In most countries, many communities have one or more prevailing micronutrient deficiencies that increase vulnerability to various disorders, such as metabolic, infectious, and non-communicable diseases. These communities should be supported with nutrient supplements—fortifying foods with vitamin D and other essential micronutrients [98], enabling them to develop a robust immune system.
Severe vitamin D deficiency and a weaker immune system is the primary cause of developing complications and deaths from sepsis and infections like SARS-CoV-2 [109]. Maintaining serum 25(OH)D concentrations above 40 ng/mL (100 nmol/L) significantly reduces microbial infections, including COVID-19 [4][110]. The mentioned targeted food fortification program is an economical and practical approach for alleviating micronutrient malnutrition in ethnic populations or even for an entire country, as has been done with iodine.
Sustained vitamin D deficiency negatively affects all body systems and increases risks for viral infections, outbreaks, and hospitalization. Thus, government and health administrators should consider nationwide educational campaigns for safe sun exposure, vitamin D supplementation, and targeted food fortification programs to strengthen the population’s immunity and keep them healthy. These acts cost less than 0.01% of one day’s hospitalization and significantly reduce healthcare costs.
10. Conclusions
Vitamin D sufficiency minimizes viral infections, outbreaks, hospitalization, deaths, and healthcare costs. Maintaining population serum 25(OH)D concentrations above 40 ng/mL (preferably above 50 ng/mL) ensures a robust immune system, curtailing the spread of infections, minimizing diseases, and reducing chronic diseases. Thus, governments, health insurance companies, and health administrators should work together and consider nationwide educational campaigns for safe sun exposure, vitamin D supplementation, and targeted food fortification programs to strengthen the population’s immunity and keep them healthy. While the efficacy of vaccines and bivalent boosters has waded due to immune evasion by Omicron mutants, the effectiveness of vitamin D sufficiency (and ivermectin) remains strong. However, vitamin D is not a panacea. Apart from preventative use, vitamin D should be used as adjunctive therapy with other established primary pharmaceuticals and the best practices, such as using antibiotics in bacterial infections. The key to protecting the vulnerable and reducing chronic disease burden in a country is not expanding hospitals and health centers and recruiting more healthcare professionals but educating the public on health preservation and maintaining a higher circulatory vitamin D concentration, especially in vulnerable communities.
This entry is adapted from the peer-reviewed paper 10.3390/nu15163623
References
- Wimalawansa, S.J. Physiological basis for using vitamin D to improve health. Biomedicines 2023, 11, doi:10.3390/biomedicines11061542.
- Wimalawansa, S.J. Commonsense approaches to minimizing risks from COVID-19. Open J Pulm Respir Med 2020, 2, 28-37, https://doi.org/10.36811/ojprm.32020.110010 doi:10.36811/ojprm.2020.110010.
- Wimalawansa, S.J. Fighting against COVID-19: Boosting the immunity with micronutrients, stress reduction, physical activity, and vitamin D. Nutrition and Food Science Journal (Sci Literature) 2020c, 3(1), 1-4.
- Zdrenghea, M.T.; Makrinioti, H.; Bagacean, C.; Bush, A.; Johnston, S.L.; Stanciu, L.A. Vitamin D modulation of innate immune responses to respiratory viral infections. Rev Med Virol 2017, 27, doi:10.1002/rmv.1909.
- Gotelli, E.; Soldano, S.; Hysa, E.; Paolino, S.; Campitiello, R.; Pizzorni, C.; Sulli, A.; Smith, V.; Cutolo, M. Vitamin D and COVID-19: Narrative Review after 3 Years of Pandemic. Nutrients 2022, 14, doi:10.3390/nu14224907.
- Xiaoyu, Z.; Payal, B.; Melissa, O.; Zanello, L.P. 1alpha,25(OH)2-vitamin D3 membrane-initiated calcium signaling modulates exocytosis and cell survival. J Steroid Biochem Mol Biol 2007, 103, 457-461, doi:10.1016/j.jsbmb.2006.11.002.
- Hollis, B.W.; Marshall, D.T.; Savage, S.J.; Garrett-Mayer, E.; Kindy, M.S.; Gattoni-Celli, S. Vitamin D3 supplementation, low-risk prostate cancer, and health disparities. J Steroid Biochem Mol Biol 2013, 136, 233-237, doi:10.1016/j.jsbmb.2012.11.012.
- Wimalawansa, S. Overcoming infections including COVID-19, by maintaining circulating 25(OH)D concentrations above 50 ng/mL. Pathology & Lab. Medicine Int. 2022, 14, 37–60.
- Wimalawansa, S.J. Vitamin D in the new millennium. Curr Osteoporos Rep 2012, 10, 4-15, doi:10.1007/s11914-011-0094-8.
- Wimalawansa, S.J. Non-musculoskeletal benefits of vitamin D. J Steroid Biochem Mol Biol 2018, 175, 60-81, doi:10.1016/j.jsbmb.2016.09.016.
- Wimalawansa, S.J. Biology of vitamin D. J steroids Horm Sci 2019, 10, 1-8, doi:10.24105/2157-7536.10.198.
- Zhang, Q.; Wang, M.; Han, C.; Wen, Z.; Meng, X.; Qi, D.; Wang, N.; Du, H.; Wang, J.; Lu, L.; et al. Intraduodenal Delivery of Exosome-Loaded SARS-CoV-2 RBD mRNA Induces a Neutralizing Antibody Response in Mice. Vaccines (Basel) 2023, 11, doi:10.3390/vaccines11030673.
- Jiang, Y.; Chen, L.; Taylor, R.N.; Li, C.; Zhou, X. Physiological and pathological implications of retinoid action in the endometrium. J Endocrinol 2018, 236, R169-R188, doi:10.1530/JOE-17-0544.
- Keane, K.N.; Cruzat, V.F.; Calton, E.K.; Hart, P.H.; Soares, M.J.; Newsholme, P.; Yovich, J.L. Molecular actions of vitamin D in reproductive cell biology. Reproduction 2017, 153, R29-R42, doi:10.1530/REP-16-0386.
- Holick, M.F. The cutaneous photosynthesis of previtamin D3: a unique photoendocrine system. J Invest Dermatol 1981, 77, 51-58, doi:10.1111/1523-1747.ep12479237.
- Haddad, J.G.; Matsuoka, L.Y.; Hollis, B.W.; Hu, Y.Z.; Wortsman, J. Human plasma transport of vitamin D after its endogenous synthesis. J Clin Invest 1993, 91, 2552-2555, doi:10.1172/JCI116492.
- Ponchon, G.; Kennan, A.L.; DeLuca, HF "Activation" of vitamin D by the liver. J Clin Invest 1969, 48, 2032-2037, doi:10.1172/JCI106168.
- Hosseinpour, F.; Wikvall, K. Porcine microsomal vitamin D(3) 25-hydroxylase (CYP2D25). Catalytic properties, tissue distribution, and comparison with human CYP2D6. J Biol Chem 2000, 275, 34650-34655, doi:10.1074/jbc.M004185200.
- Flanagan, J.N.; Young, M.V.; Persons, K.S.; Wang, L.; Mathieu, J.S.; Whitlatch, L.W.; Holick, M.F.; Chen, T.C. Vitamin D metabolism in human prostate cells: implications for prostate cancer chemoprevention by vitamin D. Anticancer Res 2006, 26, 2567-2572.
- Zhu, P.; Ren, M.; Yang, C.; Hu, Y.X.; Ran, J.M.; Yan, L. Involvement of RAGE, MAPK and NF-kappaB pathways in AGEs-induced MMP-9 activation in HaCaT keratinocytes. Exp Dermatol 2012, 21, 123-129, doi:10.1111/j.1600-0625.2011.01408.x.
- McBrearty, N.; Cho, C.; Chen, J.; Zahedi, F.; Peck, A.R.; Radaelli, E.; Assenmacher, C.A.; Pavlak, C.; Devine, A.; Yu, P.; et al. Tumor-Suppressive and Immune-Stimulating Roles of Cholesterol 25-hydroxylase in Pancreatic Cancer Cells. Mol Cancer Res 2023, 21, 228-239, doi:10.1158/1541-7786.MCR-22-0602.
- Karlgren, M.; Miura, S.; Ingelman-Sundberg, M. Novel extrahepatic cytochrome P450s. Toxicology and applied pharmacology 2005, 207, 57-61, doi:10.1016/j.taap.2004.12.022.
- Vieth, R.; Kessler, M.J.; Pritzker, K.P. Species differences in the binding kinetics of 25-hydroxyvitamin D3 to vitamin D binding protein. Can J Physiol Pharmacol 1990, 68, 1368-1371, doi:10.1139/y90-207.
- Haddad, J.G.; Hillman, L.; Rojanasathit, S. Human serum binding capacity and affinity for 25-hydroxyergocalciferol and 25-hydroxycholecalciferol. J Clin Endocrinol Metab 1976, 43, 86-91, doi:10.1210/jcem-43-1-86.
- Smith, J.E.; Goodman, D.S. The turnover and transport of vitamin D and of a polar metabolite with the properties of 25-hydroxycholecalciferol in human plasma. J Clin Invest 1971, 50, 2159-2167, doi:10.1172/JCI106710.
- Adams, J.S.; Clemens, T.L.; Parrish, J.A.; Holick, M.F. Vitamin-D synthesis and metabolism after ultraviolet irradiation of normal and vitamin-D-deficient subjects. N Engl J Med 1982, 306, 722-725, doi:10.1056/NEJM198203253061206.
- Lo, C.W.; Paris, P.W.; Clemens, T.L.; Nolan, J.; Holick, M.F. Vitamin D absorption in healthy subjects and in patients with intestinal malabsorption syndromes. Am J Clin Nutr 1985, 42, 644-649, doi:10.1093/ajcn/42.4.644.
- Heaney, R.P.; Recker, R.R.; Grote, J.; Horst, R.L.; Armas, L.A. Vitamin D(3) is more potent than vitamin D(2) in humans. J Clin Endocrinol Metab 2011, 96, E447-452, doi:10.1210/jc.2010-2230.
- Hollis, B.W.; Johnson, D.; Hulsey, T.C.; Ebeling, M.; Wagner, C.L. Vitamin D supplementation during pregnancy: double-blind, randomized clinical trial of safety and effectiveness. J Bone Miner Res 2011, 26, 2341-2357, doi:10.1002/jbmr.463.
- Mangin, M.; Sinha, R.; Fincher, K. Inflammation and vitamin D: the infection connection. Inflamm Res 2014, 63, 803-819, doi:10.1007/s00011-014-0755-z.
- Chung, M.K.; Karnik, S.; Saef, J.; Bergmann, C.; Barnard, J.; Lederman, M.M.; Tilton, J.; Cheng, F.; Harding, C.V.; Young, J.B.; et al. SARS-CoV-2 and ACE2: The biology and clinical data settling the ARB and ACEI controversy. EBioMedicine 2020, 58, 102907, doi:10.1016/j.ebiom.2020.102907.
- Wimalawansa, S.J. Public health interventions for chronic diseases: cost-benefit modelizations for eradicating chronic kidney disease of multifactorial origin (CKDmfo/ CKDu) from tropical countries. Heliyon 2019, 5, e02309, doi:10.1016/j.heliyon.2019.e02309.
- Arai, Y.; Kanda, E.; Iimori, S.; Naito, S.; Noda, Y.; Kawasaki, T.; Sato, H.; Ando, R.; Sasaki, S.; Sohara, E.; et al. The use of vitamin D analogs is independently associated with the favorable renal prognosis in chronic kidney disease stages 4-5: the CKD-ROUTE study. Clin Exp Nephrol 2017, 21, 481-487, doi:10.1007/s10157-016-1300-x.
- Oh, T.R.; Kim, C.S.; Bae, E.H.; Ma, S.K.; Han, S.H.; Sung, S.A.; Lee, K.; Oh, K.H.; Ahn, C.; Kim, S.W.; et al. Association between vitamin D deficiency and health-related quality of life in patients with chronic kidney disease from the KNOW-CKD study. PLoS One 2017, 12, e0174282, doi:10.1371/journal.pone.0174282.
- Zittermann, A.; Iodice, S.; Pilz, S.; Grant, W.B.; Bagnardi, V.; Gandini, S. Vitamin D deficiency and mortality risk in the general population: a meta-analysis of prospective cohort studies. Am J Clin Nutr 2012, 95, 91-100, doi:10.3945/ajcn.111.014779.
- Jean, G.; Souberbielle, J.C.; Chazot, C. Vitamin D in Chronic Kidney Disease and Dialysis Patients. Nutrients 2017, 9, doi:10.3390/nu9040328.
- Waziri, B.; Duarte, R.; Naicker, S. Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD): Current Perspectives. Int J Nephrol Renovasc Dis 2019, 12, 263-276, doi:10.2147/IJNRD.S191156.
- Urena-Torres, P.; Metzger, M.; Haymann, J.P.; Karras, A.; Boffa, J.J.; Flamant, M.; Vrtovsnik, F.; Gauci, C.; Froissart, M.; Houillier, P.; et al. Association of kidney function, vitamin D deficiency, and circulating markers of mineral and bone disorders in CKD. Am J Kidney Dis 2011, 58, 544-553, doi:10.1053/j.ajkd.2011.04.029.
- Kanai, G.; Fukagawa, M. [CKD-MBD (Chronic Kidney Disease-Mineral and Bone Disorder). Gene therapy for secondary hyperparathyroidism]. Clin Calcium 2010, 20, 1052-1059.
- Zelnick, L.R.; de Boer, I.H.; Kestenbaum, B.R.; Chonchol, M.; Kendrick, J. Comparative Effects of Cholecalciferol and Calcitriol on Circulating Markers of CKD Mineral Bone Disorder: A Randomized Clinical Trial. Clin J Am Soc Nephrol 2018, 13, 927-928, doi:10.2215/CJN.00480118.
- Bansal, N.; Katz, R.; Appel, L.; Denburg, M.; Feldman, H.; Go, A.S.; He, J.; Hoofnagle, A.; Isakova, T.; Kestenbaum, B.; et al. Vitamin D Metabolic Ratio and Risks of Death and CKD Progression. Kidney Int Rep 2019, 4, 1598-1607, doi:10.1016/j.ekir.2019.08.014.
- Vieth, R. Why the optimal requirement for Vitamin D3 is probably much higher than what is officially recommended for adults. J Steroid Biochem Mol Biol 2004, 89-90, 575-579, doi:10.1016/j.jsbmb.2004.03.038.
- Nykjaer, A.; Dragun, D.; Walther, D.; Vorum, H.; Jacobsen, C.; Herz, J.; Melsen, F.; Christensen, E.I.; Willnow, T.E. An endocytic pathway essential for renal uptake and activation of the steroid 25-(OH) vitamin D3. Cell 1999, 96, 507-515, doi:10.1016/s0092-8674(00)80655-8.
- Marzolo, M.P.; Farfan, P. New insights into the roles of megalin/LRP2 and the regulation of its functional expression. Biol Res 2011, 44, 89-105, doi:10.4067/S0716-97602011000100012.
- Wimalawansa, S.J. Rapidly Increasing Serum 25(OH)D Boosts the Immune System, against Infections-Sepsis and COVID-19. Nutrients 2022, 14, doi:10.3390/nu14142997.
- Ianevski, A.; Zusinaite, E.; Shtaida, N.; Kallio-Kokko, H.; Valkonen, M.; Kantele, A.; Telling, K.; Lutsar, I.; Letjuka, P.; Metelitsa, N.; et al. Low Temperature and Low UV Indexes Correlated with Peaks of Influenza Virus Activity in Northern Europe during 2010(-)2018. Viruses 2019, 11, doi:10.3390/v11030207.
- Imai, C.M.; Halldorsson, T.I.; Eiriksdottir, G.; Cotch, M.F.; Steingrimsdottir, L.; Thorsdottir, I.; Launer, L.J.; Harris, T.; Gudnason, V.; Gunnarsdottir, I. Depression and serum 25-hydroxyvitamin D in older adults living at northern latitudes - AGES-Reykjavik Study. J Nutr Sci 2015, 4, e37, doi:10.1017/jns.2015.27.
- Devaraj, S.; Jialal, G.; Cook, T.; Siegel, D.; Jialal, I. Low vitamin D levels in Northern American adults with the metabolic syndrome. Horm Metab Res 2011, 43, 72-74, doi:10.1055/s-0030-1268485.
- Reichrath, J.; Saternus, R.; Vogt, T. Challenge and perspective: the relevance of ultraviolet (UV) radiation and the vitamin D endocrine system (VDES) for psoriasis and other inflammatory skin diseases. Photochem Photobiol Sci 2017, 16, 433-444, doi:10.1039/c6pp00280c.
- Premkumar, M.; Sable, T.; Dhanwal, D.; Dewan, R. Vitamin D homeostasis, bone mineral metabolism, and seasonal affective disorder during 1 year of Antarctic residence. Archives of osteoporosis 2013, 8, 129, doi:10.1007/s11657-013-0129-0.
- Saraiva, G.L.; Cendoroglo, M.S.; Ramos, L.R.; Araujo, L.M.; Vieira, J.G.; Kunii, I.; Hayashi, L.F.; Correa, M.P.; Lazaretti-Castro, M. Influence of ultraviolet radiation on the production of 25 hydroxyvitamin D in the elderly population in the city of Sao Paulo. Osteoporos Int 2005, 16, 1649-1654, doi:10.1007/s00198-005-1895-3.
- Pletz, M.W.; Terkamp, C.; Schumacher, U.; Rohde, G.; Schutte, H.; Welte, T.; Bals, R.; Group, C.A.-S. Vitamin D deficiency in community-acquired pneumonia: low levels of 1,25(OH)2 D are associated with disease severity. Respir Res 2014, 15, 53, doi:10.1186/1465-9921-15-53.
- Ginde, A.A.; Mansbach, J.M.; Camargo, C.A., Jr. Vitamin D, respiratory infections, and asthma. Curr Allergy Asthma Rep 2009, 9, 81-87, doi:10.1007/s11882-009-0012-7.
- Czaja, A.J. Factoring the intestinal microbiome into the pathogenesis of autoimmune hepatitis. World J Gastroenterol 2016, 22, 9257-9278, doi:10.3748/wjg.v22.i42.9257.
- Jin, D.; Wu, S.; Zhang, Y.G.; Lu, R.; Xia, Y.; Dong, H.; Sun, J. Lack of Vitamin D Receptor Causes Dysbiosis and Changes the Functions of the Murine Intestinal Microbiome. Clin Ther 2015, 37, 996-1009 e1007, doi:10.1016/j.clinthera.2015.04.004.
- Zhou, Y.F.; Luo, B.A.; Qin, L.L. The association between vitamin D deficiency and community-acquired pneumonia: A meta-analysis of observational studies. Medicine (Baltimore) 2019, 98, e17252, doi:10.1097/MD.0000000000017252.
- Laplana, M., Royo, J.L, Fibla, J. Vitamin D Receptor polymorphisms and risk of enveloped virus infection: A meta-analysis. Gene 2018, 678, 384-394, doi:10.1016/j.gene.2018.08.017.
- Platitsyna, N.G.; Bolotnova, T.V. [Vitamin D deficiency as a risk factor for chronic non-infectious diseases.]. Adv Gerontol 2017, 30, 873-879.
- Grant, W.B.; Lahore, H.; McDonnell, S.L.; Baggerly, C.A.; French, C.B.; Aliano, J.L.; Bhattoa, H.P. Evidence that Vitamin D Supplementation Could Reduce Risk of Influenza and COVID-19 Infections and Deaths. Nutrients 2020, 12, 1626, doi:10.3390/nu12040988.
- Eroglu, C.; Demir, F.; Erge, D.; Uysal, P.; Kirdar, S.; Yilmaz, M.; Kurt Omurlu, I. The relation between serum vitamin D levels, viral infections and severity of attacks in children with recurrent wheezing. Allergol Immunopathol (Madr) 2019, 47, 591-597, doi:10.1016/j.aller.2019.05.002.
- Arihiro, S.; Nakashima, A.; Matsuoka, M.; Suto, S.; Uchiyama, K.; Kato, T.; Mitobe, J.; Komoike, N.; Itagaki, M.; Miyakawa, Y.; et al. Randomized Trial of Vitamin D Supplementation to Prevent Seasonal Influenza and Upper Respiratory Infection in Patients With Inflammatory Bowel Disease. Inflamm Bowel Dis 2019, 25, 1088-1095, doi:10.1093/ibd/izy346.
- Jolliffe, D.A.; Greiller, C.L.; Mein, C.A.; Hoti, M.; Bakhsoliani, E.; Telcian, A.G.; Simpson, A.; Barnes, N.C.; Curtin, J.A.; Custovic, A.; et al. Vitamin D receptor genotype influences risk of upper respiratory infection. Br J Nutr 2018, 120, 891-900, doi:10.1017/S000711451800209X.
- Wagner, C.L.; Hollis, B.W.; Kotsa, K.; Fakhoury, H.; Karras, S.N. Vitamin D administration during pregnancy as prevention for pregnancy, neonatal and postnatal complications. Rev Endocr Metab Disord 2017, 18, 307-322, doi:10.1007/s11154-017-9414-3.
- Morris, S.K.; Pell, L.G.; Rahman, M.Z.; Dimitris, M.C.; Mahmud, A.; Islam, M.M.; Ahmed, T.; Pullenayegum, E.; Kashem, T.; Shanta, S.S.; et al. Maternal vitamin D supplementation during pregnancy and lactation to prevent acute respiratory infections in infancy in Dhaka, Bangladesh (MDARI trial): protocol for a prospective cohort study nested within a randomized controlled trial. BMC Pregnancy Childbirth 2016, 16, 309, doi:10.1186/s12884-016-1103-9.
- Wimalawansa, S.J., Polonowita, A. Boosting immunity with vitamin D for preventing complications and deaths from COVID-19. In Proceedings of the COVID 19: Impact, mitigation, opportunities and building resilience “From adversity to serendipity,” perspectives of global relevance based on research, experience and successes in combating COVID-19 in Sri Lanka, Colombo, Sri Lanka, 2021; pp. 171-198.
- Kone-Paut, I.; Cimaz, R. Is it Kawasaki shock syndrome, Kawasaki-like disease or pediatric inflammatory multisystem disease? The importance of semantic in the era of COVID-19 pandemic. RMD Open 2020, 6, doi:10.1136/rmdopen-2020-001333.
- Stagi, S.; Rigante, D.; Lepri, G.; Matucci Cerinic, M.; Falcini, F. Severe vitamin D deficiency in patients with Kawasaki disease: a potential role in the risk to develop heart vascular abnormalities? Clin Rheumatol 2016, 35, 1865-1872, doi:10.1007/s10067-015-2970-6.
- Wimalawansa, S.J. Multisystem Inflammatory Syndrome (MIS) and Kawasaki-like syndrome in children with severe vitamin D deficiency. LinkedIn 2021.
- Pouletty, M.; Borocco, C.; Ouldali, N.; Caseris, M.; Basmaci, R.; Lachaume, N.; Bensaid, P.; Pichard, S.; Kouider, H.; Morelle, G.; et al. Paediatric multisystem inflammatory syndrome temporally associated with SARS-CoV-2 mimicking Kawasaki disease (Kawa-COVID-19): a multicentre cohort. Ann Rheum Dis 2020, 79, 999-1006, doi:10.1136/annrheumdis-2020-217960.
- Adams, J.S., Modlin, R.L, Diz, MM, Barnes, P.F. Potentiation of the macrophage 25-hydroxyvitamin D-1-hydroxylation reaction by human tuberculous pleural effusion fluid. J Clin Endocrinol Metab 1989, 69, 457-460, doi:10.1210/jcem-69-2-457.
- Ahorsu, D.K.; Imani, V.; Lin, C.Y.; Timpka, T.; Brostrom, A.; Updegraff, J.A.; Arestedt, K.; Griffiths, M.D.; Pakpour, A.H. Associations Between Fear of COVID-19, Mental Health, and Preventive Behaviours Across Pregnant Women and Husbands: An Actor-Partner Interdependence Modelling. Int J Ment Health Addict 2022, 20, 68-82, doi:10.1007/s11469-020-00340-x.
- Aloia, J.F.; Li-Ng, M. Re: epidemic influenza and vitamin D. Epidemiol Infect 2007, 135, 1095-1096; author reply 1097-1098, doi:10.1017/S0950268807008308.
- Fleming, D.M.; Elliot, A.J. Epidemic influenza and vitamin D. Epidemiol Infect 2007, 135, 1091-1092; author reply 1092-1095, doi:10.1017/S0950268807008291.
- Wimalawansa, S.J. Controlling chronic diseases and acute infections with vitamin D sufficiency. Nutrients 2023, 15, doi:10.3390/nu15163623.
- Dawodu, A., Saadi, H.F, Bekdache, G, Javed, Y, Altaye, M, Hollis, B.W. Randomized controlled trial (RCT) of vitamin D supplementation in pregnancy in a population with endemic vitamin D deficiency. J Clin Endocrinol Metab 2013, 98, 2337-2346, doi:10.1210/jc.2013-1154.
- Camargo, C.A., Jr.; Ganmaa, D.; Frazier, A.L.; Kirchberg, F.F.; Stuart, J.J.; Kleinman, K.; Sumberzul, N.; Rich-Edwards, J.W. Randomized trial of vitamin D supplementation and risk of acute respiratory infection in Mongolia. Pediatrics 2012, 130, e561-567, doi:10.1542/peds.2011-3029.
- Bergman, P.; Norlin, A.C.; Hansen, S.; Rekha, R.S.; Agerberth, B.; Bjorkhem-Bergman, L.; Ekstrom, L.; Lindh, J.D.; Andersson, J. Vitamin D3 supplementation in patients with frequent respiratory tract infections: a randomised and double-blind intervention study. BMJ Open 2012, 2, doi:10.1136/bmjopen-2012-001663.
- Hutchinson, M.S.; Grimnes, G.; Joakimsen, R.M.; Figenschau, Y.; Jorde, R. Low serum 25-hydroxyvitamin D levels are associated with increased all-cause mortality risk in a general population: the Tromso study. Eur J Endocrinol 2010, 162, 935-942, doi:10.1530/EJE-09-1041.
- Murdoch, D.R.; Slow, S.; Chambers, S.T.; Jennings, L.C.; Stewart, A.W.; Priest, P.C.; Florkowski, C.M.; Livesey, J.H.; Camargo, C.A.; Scragg, R. Effect of vitamin D3 supplementation on upper respiratory tract infections in healthy adults: the VIDARIS randomized controlled trial. JAMA 2012, 308, 1333-1339, doi:10.1001/jama.2012.12505.
- Manaseki-Holland, S., Maroof, Z, Bruce, J, Mughal, MZ, ; Masher, M.I., Bhutta, ZA, Walraven, G, Chandramohan, D. Effect on the incidence of pneumonia of vitamin D supplementation by quarterly bolus dose to infants in Kabul: a randomised controlled superiority trial. Lancet 2012, 379, 1419-1427, doi:10.1016/S0140-6736(11)61650-4.
- Martineau, A.R.; Timms, P.M.; Bothamley, G.H.; Hanifa, Y.; Islam, K.; Claxton, A.P.; Packe, G.E.; Moore-Gillon, J.C.; Darmalingam, M.; Davidson, R.N.; et al. High-dose vitamin D(3) during intensive-phase antimicrobial treatment of pulmonary tuberculosis: a double-blind randomised controlled trial. Lancet 2011, 377, 242-250, doi:10.1016/S0140-6736(10)61889-2.
- Hollis, B.W. Short-term and long-term consequences and concerns regarding valid assessment of vitamin D deficiency: comparison of recent food supplementation and clinical guidance reports. Curr Opin Clin Nutr Metab Care 2011, 14, 598-604, doi:10.1097/MCO.0b013e32834be798.
- Sanders, K.M.; Stuart, A.L.; Williamson, E.J.; Simpson, J.A.; Kotowicz, M.A.; Young, D.; Nicholson, G.C. Annual high-dose oral vitamin D and falls and fractures in older women: a randomized controlled trial. JAMA 2010, 303, 1815-1822, doi:10.1001/jama.2010.594.
- Heaney, R.; Armas, L.; Shary, J.; Bell, N.; Binkley, N.; Hollis, B. 25-Hydroxylation of vitamin D3: relation to circulating vitamin D3 under various input conditions. Am J Clin Nutr 2008, 87, 1738-1742, doi:10.1093/ajcn/87.6.1738.
- Greer, F.R.; Hollis, B.W.; Napoli, J.L. High concentrations of vitamin D2 in human milk associated with pharmacologic doses of vitamin D2. J Pediatr 1984, 105, 61-64, doi:10.1016/s0022-3476(84)80361-3.
- Greer, F.R.; Hollis, B.W.; Cripps, D.J.; Tsang, R.C. Effects of maternal ultraviolet B irradiation on vitamin D content of human milk. J Pediatr 1984, 105, 431-433, doi:10.1016/s0022-3476(84)80021-9.
- Ziegler, E.E.; Hollis, B.W.; Nelson, S.E.; Jeter, J.M. Vitamin D deficiency in breastfed infants in Iowa. Pediatrics 2006, 118, 603-610, doi:10.1542/peds.2006-0108.
- Wagner, C.L.; Greer, F.R.; American Academy of Pediatrics Section on, B.; American Academy of Pediatrics Committee on, N. Prevention of rickets and vitamin D deficiency in infants, children, and adolescents. Pediatrics 2008, 122, 1142-1152, doi:10.1542/peds.2008-1862.
- Hollis, B.W.; Wagner, C.L. Vitamin D requirements during lactation: high-dose maternal supplementation as therapy to prevent hypovitaminosis D for both the mother and the nursing infant. Am J Clin Nutr 2004, 80, 1752S-1758S, doi:10.1093/ajcn/80.6.1752S.
- Hollis, B.W.; Pittard, W.B., 3rd. Evaluation of the total fetomaternal vitamin D relationships at term: evidence for racial differences. J Clin Endocrinol Metab 1984, 59, 652-657, doi:10.1210/jcem-59-4-652.
- Hillman, L.S.; Haddad, J.G. Human perinatal vitamin D metabolism. I. 25-Hydroxyvitamin D in maternal and cord blood. J Pediatr 1974, 84, 742-749, doi:10.1016/s0022-3476(74)80024-7.
- Heyden, E.L.; Wimalawansa, S.J. Vitamin D: Effects on human reproduction, pregnancy, and fetal well-being. J Steroid Biochem Mol Biol 2018, 180, 41-50, doi:10.1016/j.jsbmb.2017.12.011.
- Richardson, S.; Hirsch, J.S.; Narasimhan, M.; Crawford, J.M.; McGinn, T.; Davidson, K.W.; the Northwell, C.-R.C.; Barnaby, D.P.; Becker, L.B.; Chelico, J.D.; et al. Presenting Characteristics, Comorbidities, and Outcomes Among 5700 Patients Hospitalized With COVID-19 in the New York City Area. JAMA 2020, 323, 2052-2059, doi:10.1001/jama.2020.6775.
- Kurylowicz, A.; Bednarczuk, T.; Nauman, J. [The influence of vitamin D deficiency on cancers and autoimmune diseases development]. Endokrynol Pol 2007, 58, 140-152.
- Quraishi, S.A.; Bittner, E.A.; Blum, L.; Hutter, M.M.; Camargo, C.A., Jr. Association between preoperative 25-hydroxyvitamin D level and hospital-acquired infections following Roux-en-Y gastric bypass surgery. JAMA Surg 2014, 149, 112-118, doi:10.1001/jamasurg.2013.3176.
- Quraishi, S.A.; De Pascale, G.; Needleman, J.S.; Nakazawa, H.; Kaneki, M.; Bajwa, E.K.; Camargo, C.A., Jr.; Bhan, I. Effect of Cholecalciferol Supplementation on Vitamin D Status and Cathelicidin Levels in Sepsis: A Randomized, Placebo-Controlled Trial. Crit Care Med 2015, 43, 1928-1937, doi:10.1097/CCM.0000000000001148.
- Borsche, L.; Glauner, B.; von Mendel, J. COVID-19 Mortality Risk Correlates Inversely with Vitamin D3 Status, and a Mortality Rate Close to Zero Could Theoretically Be Achieved at 50 ng/mL 25(OH)D3: Results of a Systematic Review and Meta-Analysis. Nutrients 2021, 13, doi:10.3390/nu13103596.
- Wimalawansa, S.J. Effective and practical ways to overcome vitamin D deficiency. J Family Med Community Health 2021, 8, 1-8, doi:https://www.jscimedcentral.com/FamilyMedicine/familymedicine-7-1170.pdf.
- Shrestha, N.K.; Shrestha, P.; Burke, P.C.; Nowacki, A.S.; Terpeluk, P.; Gordon, S.M. Coronavirus Disease 2019 Vaccine Boosting in Previously Infected or Vaccinated Individuals. Clin Infect Dis 2022, 75, 2169-2177, doi:10.1093/cid/ciac327.
- Uraki, R.; Ito, M.; Furusawa, Y.; Yamayoshi, S.; Iwatsuki-Horimoto, K.; Adachi, E.; Saito, M.; Koga, M.; Tsutsumi, T.; Yamamoto, S.; et al. Humoral immune evasion of the omicron subvariants BQ.1.1 and XBB. Lancet Infect Dis 2023, 23, 30-32, doi:10.1016/S1473-3099(22)00816-7.
- Dingemans, J.; van der Veer, B.; Gorgels, K.M.F.; Hackert, V.; den Heijer, C.D.J.; Hoebe, C.; Savelkoul, P.H.M.; van Alphen, LB Investigating SARS-CoV-2 breakthrough infections per variant and vaccine type. Front Microbiol 2022, 13, 1027271, doi:10.3389/fmicb.2022.1027271.
- Wimalawansa, S.J. Controlling COVID-19 pandemic with cholecalciferol. World J Adv, Heathcare Res. 2020, 5, 155-165, doi:10.17605/OSF.IO/7UQ6P.
- Borel, P.; Caillaud, D.; Cano, N.J. Vitamin D bioavailability: state of the art. Critical reviews in food science and nutrition 2015, 55, 1193-1205, doi:10.1080/10408398.2012.688897.
- Johansson, H.; Oden, A.; Kanis, J.; McCloskey, E.; Lorentzon, M.; Ljunggren, O.; Karlsson, M.K.; Thorsby, P.M.; Tivesten, A.; Barrett-Connor, E.; et al. Low serum vitamin D is associated with increased mortality in elderly men: MrOS Sweden. Osteoporos Int 2012, 23, 991-999, doi:10.1007/s00198-011-1809-5.
- Biesalski, H.K.; Aggett, P.J.; Anton, R.; Bernstein, P.S.; Blumberg, J.; Heaney, R.P.; Henry, J.; Nolan, J.M.; Richardson, D.P.; van Ommen, B.; et al. 26th Hohenheim Consensus Conference, September 11, 2010 Scientific substantiation of health claims: evidence-based nutrition. Nutrition 2011, 27, S1-20, doi:10.1016/j.nut.2011.04.002.
- Tsai, F.; Coyle, W.J. The microbiome and obesity: is obesity linked to our gut flora? Curr Gastroenterol Rep 2009, 11, 307-313, doi:10.1007/s11894-009-0045-z.
- Dai, Q.; Zhu, X.; Manson, J.E.; Song, Y.; Li, X.; Franke, A.A.; Costello, R.B.; Rosanoff, A.; Nian, H.; Fan, L.; et al. Magnesium status and supplementation influence vitamin D status and metabolism: results from a randomized trial. Am J Clin Nutr 2018, 108, 1249-1258, doi:10.1093/ajcn/nqy274.
- Magnesium and vitamin D deficiency as a potential cause of immune dysfunction, cytokine storm and disseminated intravascular coagulation in covid-19 patients.
- Aslam, S.; Danziger-Isakov, L.; Mehra, M.R. COVID-19 vaccination immune paresis in heart and lung transplantation. J Heart Lung Transplant 2021, 40, 763-766, doi:10.1016/j.healun.2021.04.018.
- Arabi, YM; Fowler, R.; Hayden, F.G. Critical care management of adults with community-acquired severe respiratory viral infection. Intensive Care Med 2020, 46, 315-328, doi:10.1007/s00134-020-05943-5.
