Sepsis and septic shock continue to be a leading cause of death worldwide. Sepsis was previously thought to be an overwhelming, systemic, proinflammatory response to infection, which was followed by a phase of immunosuppression. New paradigms suggest that the proinflammatory and immunosuppression phases occurs simultaneously, and the pathophysiology begind the disease complex is not only explained by the pathogen’s type, load and virulence, but to a large extend also by host’s dysregulated response to infection. Many of these dysregulated host immune responses that occurs in sepsis are also targets of hyperbaric oxygen (HBO2) treatment. HBO2 treatment has been shown to improve survival in clinical studies on patients with necrotizing soft tissue infections as well as experimental sepsis models. Inflammation and oxygen-sensing pathways are connected on the cellular level in a self-reinforcing and detrimental manner in inflammatory conditions, which may be interrupted when intervening with HBO2 treatment. HBO2 treatment acts to maintain homeostasis by protecting the host from collateral tissue damage during resistance to infection by reducing neutrophil extracellular traps, inhibiting neutrophil adhesion to vascular endothelium, reducing proinflammatory cytokines, and halting the Warburg effect, while also aiding the host in tolerance to infection by reducing iron-mediated injury and upregulating anti-inflammatory measures.
1. Neutrophil Mediated Responses in Sepsis
Neutrophils play a central role in the resistance to infection by removing invading pathogens through phagocytosis [
29]. Other important functional responses that neutrophils use in host defense include cell migration, degranulation, oxidative bursts thought the synthesis of reactive oxygen species (ROS) and
generation of neutrophil extracellular traps (NETs) [30]. NETs can entrap pathogens and thereby contribute to pathogen elimination. However, uncontrolled NETs’ release can also contribute to collateral damage to host tissue and thrombosis by serving as a scaffold for the entrapment and aggregation of platelets and erythrocytes [28]. Patients with sepsis have elevated NET levels in their blood, which are linked to organ dysfunction [32]. HBO2 treatment has a complex effect on the host’s oxidative status, which is likely to be dependent on both dose and timing, as well as tissue oxygenation at the time of application. HBO2 has been shown in cells from healthy volunteers to reduce ROS production by neutrophils after two and three HBO2 treatment sessions [33]. The same study found no change in neutrophil phagocytic activity, circulating cytokines, or systemic oxidative stress, as indicated by plasma malondialdehyde concentrations [33]. Similar results were found in a study evaluating the effects of HBO2 treatment ex vivo on the activity of neutrophils harvested from severely injured patients. HBO2 treatment significantly decreased neutrophil ROS production, which was also linked to a decrease in the release of neutrophil extracellular traps (NETs) in control cells and to a lesser extent in cells from injured individuals [32]. HBO2 did not influence neutrophil chemotaxis or apoptosis [32].2. Endothelial Dysfunction in Sepsis
Sepsis is associated with severe endothelial cell dysfunction. Cytokines and ROS induce glycocalyx shedding in inflammation and sepsis, exposing adhesion molecules and initiating leukocyte adhesion, which leads to transmigration to tissues. Once they have entered the tissues, leukocytes can release inflammatory mediators and reactive molecules to destroy pathogens, but at the same time potentially cause tissue damage [
34]. Given the central role of ROS and reactive nitrogen species, HBO
2 treatment may modulate the cascade of events that results in organ failure caused by endothelial cell dysfunction. Indeed, HBO
2 treatment has been demonstrated to mediate inhibition of neutrophil adhesion to vascular endothelium [
37]. The effect has been shown to be specific to β2-integrin class [
35,
38], and it occurs because hyperoxia increases the activity of NOS (nitric oxide synthases) and MPO (myeloperoxidase) in neutrophils, resulting in the release of NO (nitric oxide)-derived oxidants, that mediate excessive S-nitrosylation of β-actin, a cytoskeleton actin in neutrophils required for β2-integrin clustering. It is a localized process occurring only within neutrophils, probably because of a scarcity of myeloperoxidase [
39]. On the endothelial side, a prospective study of patients with sepsis caused by necrotizing soft tissue infections showed that HBO
2 treatment increased soluble ICAM-1 (sICAM-1) and the effect was more pronounced in patients with septic shock. Low baseline sICAM-1 was an independent risk factor of 90-day mortality and was associated with severity of disease [
22]. HBO
2 treatment might modulate endothelial shedding of ICAM-1 and reduce the inflammation on the endothelium. This is in line with an in vivo study demonstrating that HBO
2 treatment reduces ICAM-1 on vascular endothelium under infectious conditions [
40].
3. The Cytokine Mediated Inflammatory Response in Sepsis
Substantial in vitro and in vivo evidence suggests that NF-κB (Nuclear Factor kappa-B) plays a critical role in sepsis [
18]. NF-κB is a transcription factor family that is involved in a variety of biological responses that underpin the phenotypic outcomes of inflammation, immune response modulation, cell growth, proliferation, apoptosis, and aspects of differentiation and development. Increased and/or prolonged activation of NF-κB result in the overexpression of mediator proteins and may account for some of the deleterious effects seen in sepsis [
18]. NF-κB is a redox-sensitive transcription factor, and exposure to oxidants such as hydrogen peroxide causes nuclear translocation of NF-κB in certain cells [
44]. Interestingly, HBO
2 treatment has been shown to decrease the expression of NF-κB at protein level in both an LPS (liposaccharide) model of sepsis, neuroinflammation, healthy cells, and cancer cells [
45,
46,
47], preventing the production of inflammatory cytokines and pulling in an anti-inflammatory direction under ongoing stress [
46,
48]. HBO
2 treatment has been shown to reduce NF-κB controlled proinflammatory cytokines such as IL-1, IL-6, and TNF-α both in vitro and in vivo [
49], and HBO
2 treatment was associated with a decrease in IL-6 and G-CSF in plasma from Group A-Streptococcus NSTI sepsis patients [
23]. However, some studies have not found any changes in cytokine levels after HBO
2 treatment [
33]. A systematic review of 58 papers on inflammatory markers in human tissue in response to HBO
2 treatment found an inhibiting effect on NF-κB, IL-1β, IL-6, and IL-8, as well as an anti-inflammatory state in general [
48].
4 . Switching Immune Cell Metabolism toward Glycolysis in Sepsis—The Warburg Effect
The energy required for normal cellular activity is normally produced in the cytosol by glycolysis or, to a much greater extent, oxidative phosphorylation. Otto Warburg discovered that cancer cells produced energy via glycolysis rather than oxidative phosphorylation [
50]. The Warburg Effect is increasingly recognized as an essential regulator of innate and adaptive immunity and may be as much a hallmark of sepsis as it is of cancer [
51,
52,
53,
54]. The Warburg effect may help to regulate innate immune functions in activated immune cells like macrophages, dendritic cells, and T cells by providing a readily available source of energy for phagocytosis, oxidative burst, and biosynthetic precursors to divide and produce cytokines, and thus acts as a way to regulate host resistance to infection [
55]. Glycolysis continues in both inflammation and cancer despite adequate oxygen delivery to the tissues. A proposed mechanism behind this phenomena is that pyruvate is kept in the cytoplasm, where it can be reduced to lactate even in the presence of oxygen, preventing it from being used in the tricarboxylic acid cycle [
53]. Excessive pro-inflammatory glycolytic drive may have a negative impact on host cell homeostasis. HBO
2 treatment can stimulate changes in cellular energy metabolism thereby halting the Warburg effect [
60]. Because sepsis and cancer are thought to have similar biochemical events in terms of the Warburg effect, studies on HBO
2 treatments’ effect on the Warburg effect in carcinogenic illnesses may be generalized to sepsis situations. Hypoxia indicible factor-1 (HIF-1) downregulation was required for HBO
2 treatment to suppress the Warburg effect in hypoxic cancer cells, according to in vitro research on non-small cell lung cancer cell lines [
61]. Another study found that HBO
2 reduced oxidative phosphorylation during both the initial response phase and the recovery phase of energy production. HBO
2 also downregulated ribosomal protein S6 kinase, a target of the mTOR pathway [
60]. Furthermore, a few studies have shown that a ketogenic diet combined with HBO
2 treatment can limit tumor growth in experimental models of metastatic cancer by blocking the Warburg effect [
61,
62,
63].
5 . Iron metabolism in the pathogenesis of sepsis
In severe bacterial infections, invading bacteria are frequently transferred from the local infectious site to the bloodstream, where they can cause hemolysis, which results in the oxidation of extracellular hemoglobin and the accumulation of labile heme in plasma. [6, 8]. To increase their tolerance for infection, the host has defenses against the cytotoxic effects of heme. HO-1 (Heme oxygenase-1) catabolizes heme and breaks it down into biliverdin, iron (Fe), and carbon monoxide [65]. The expression of HO-1 is dependent on the cell type, cellular microenvironment, intensity and duration of stimuli exposure, and is regulated by a panel of redox-sensitive transcription factors, including HIF-1α and NF-κB [69]. In numerous studies and circumstances, HBO2 treatment has been linked to a protective impact of enhanced HO-1 activity [70-75]. Most pertinent to the aim of this paper is that levels of HO-1 have been shown to increase in a subgroup of NSTI patients with septic shock in response to HBO2 treatment [24]. Other authors have likewise demonstrated an effect of HBO2 treatment on HO-1 with a 30-fold increase in lymphocytes of healthy volunteers 24 h after HBO2 treatment [73]. The beneficial effects of HO-1 stimulation in sepsis have been demonstrated in a lipopolysaccharide-induced model, demonstrating that HO-1 induction reduces acute lung injury [70].
6 . The Anti-Inflammatory Response in Sepsis
Specific anti-inflammatory cytokines balance out the strong proinflammatory response to restore immunological balance and increase host resistance to infection [27]. Proinflammatory cytokines, anti-inflammatory cytokines, and soluble inhibitors of proinflammatory cytokines make up the cytokine network, and a tightly controlled balance between these three groups of cytokines is essential for preventing excessive, tissue-damaging inflammation during the thorough removal of invading pathogens [76, 77]. IL-10 (interleukin-10), IL-4 (interleukin-4), and TGF-β (tumor growth factor beta) are the three best described anti-inflammatory regulators operating in sepsis [78]. There have been few investigations on the influence of HBO2 medication on the production of anti-inflammatory cytokines in sepsis, with inconsistent results. One clinical study found no effect of HBO2 treatment on IL-10 in septic patients after one or three treatment sessions [23]. In a cecal ligation and puncture-induced sepsis model macrophages isolated from HBO2-treated mice demonstrated enhanced IL-10 secretion as compared with controls, and IL-10 deficiency mice were not protected from sepsis mortality by IL-10 expression [83]. A protective effect of IL-10 elevation in response to HBO2 treatment has also been demonstrated in another model [84]. Animal studies have found that HBO2 treatment decreases the level of TGF-β messengers and proteins in several conditions [85-88], while IL-4 is upregulated in response to HBO2 treatment [89-91]. This upregulation of IL-4 was confirmed in a systematic review on cytokine responses to HBO2 in human tissue, whereas the authors found no effect on IL-10 and a possible reducing effect on TGF-β [48].
7 . Hypoxia in the pathogenesis of sepsis
During an infection, tissue hypoxia is caused by vascular dysfunction and increased oxygen consumption due to increased metabolic activity of immune cells. Hypoxia acts as a stressor in cells, activating NF-κB, which then upregulates hypoxia-inducible factors (HIFs) and the cellular hypoxia response in a positive feedback loop [92, 93]. This might culminate in reinforcing pathophysiological effects in which both hypoxia and inflammation contribute to disease [94]. It has been revealed that HIF-1 may be a critical determinant of the sepsis phenotype due to its association with the production of pro-inflammatory cytokines, which results in the clinical manifestation of sepsis symptoms such as tachycardia, hypotension, and hypothermia [98, 99]. Changes in oxygen availability, rather than constant hypoxia or hyperoxia, have a more dominant effect on HIF-1α expression, making HBO2 treatment a potent regulator of HIF expression [102-104]. The effect of HBO2 treatment on HIF is multi-faceted and may occur both at the transcriptional and post-transcriptional level mediated by the actions of oxygen and reactive oxygen species. The treatment-mediated oxygen supply will result in the O2-sensitive hydroxylation of HIF-1α proteins and its inactivation via the actions of PHD and FIH. The exact mechanism by which oxygen sensing regulates the NF-κB pathway is still a matter of debate [105-107]. Both PHDs and FIH have been identified as playing a role in the control of NF-κB activity in hypoxia, either by directly impacting IKKβ (inhibitor of nuclear factor kappa B kinase subunit beta) activity, or through hydroxylase activity, ultimately leading to hindered tyrosine phosphorylation of IκBα (NF-kappa-B inhibitor alpha), and the continuous binding of this inhibitory protein [106]. When HBO2 treatment is administered in hypoxic inflammatory conditions, the regulation of HIF-1α will primarily be driven by increased oxygen delivery, which causes HIF-1α to be hydroxylated and degraded via the actions of PHD, thereby decreasing the expression of HIF-1α proteins [95]. This is in line with the previous observation that HBO2 administrated to non-inflamed well-oxygenated tissue causes an increase in HIF-1α protein levels. However, when given to inflamed and ischemic tissue that overexpresses HIF-1α, the overexpressed HIF-1α is reduced. [14]. The oxygen-mediated activation of PHD and HIF will leave NF-κB bound to its inhibitory IκBα preventing its target genes to be transcribed [106]. As a result, in septic conditions, HBO2 treatment may break the positive feedback loop mechanism by which hypoxia induces inflammation and inflammation induces hypoxia, thereby preventing the damage caused by this cycle (Figure 2) [94].
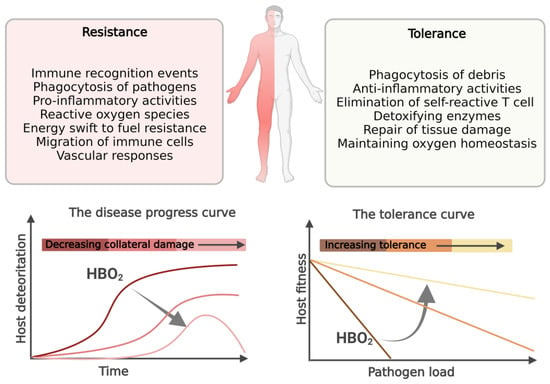
In short, the host effector mechanisms discussed above that act to increase resistance to infection and fight the invading pathogen may also reduce tolerance to infection by causing self-harm [
7], and the HBO
2 treatment acts to counterbalance collateral damage during resistance and increasing tolerance, as shown in
Figure 1.
Figure 1. The division of host defense mechanisms in infection resistance and tolerance. Host resistance effector mechanisms that restrict and contain invading pathogens may be self-harming. Disease tolerance is defined as the host’s ability to limit damage and maintain health in the face of increasing pathogen burden. The tolerance curve: in this linear relationship, hosts with steep negative slopes lose health as pathogen loads increase, whereas hosts with shallow slopes maintain relatively higher levels of health even as pathogen burden increases. We propose that HBO2 treatment can help the host immune system by increasing tolerance and preventing self-damage during resistance.
This entry is adapted from the peer-reviewed paper 10.3390/biom13081228