Specifically, the cation channel of sperm (CatSper) is a sperm-specific, pH-sensitive, and Ca
2+-permeable ion channel [
7]. Crucially, this channel is responsible for the predominant Ca
2+ entry in mammalian sperm and is involved in nearly every event by which sperm acquire their fertilizing capability. Additionally, ion channels are capable of transporting ions faster than transporters. This allows sperm to respond quickly to guidance cues within the female reproductive tract. Consequently, CatSper enables the translation of large changes in the microenvironment into changes of [Ca
2+]
i [
8]. Although fertilization is at the center of creating new life, it is still a long way from being fully understood. A better understanding of the CatSper channel is important, not only to advance knowledge of the cause of male infertility but also to inspire improvement in the development of male contraceptives. On one hand, the knockout of genes encoding the CatSper channel in male mice, as well as genetic mutations in CatSper genes in humans, lead to male infertility and the inability of sperm to undergo hyperactivation and to penetrate oocytes. On the other hand, CatSper plays a pivotal role in responding to multiple chemical cues, including physiological chemicals (such as progesterone [P4] and prostaglandins [PGs]), and synthetic and natural chemicals (such as medicines and endocrine disrupting chemicals [EDCs]). Therefore, CatSper is also a pivotal polymodal chemosensor in mammalian sperm [
9].
2. Overview of CatSper
CatSper is located in the flagellar principal piece [
7]. The CatSper complex consists of four pore-forming alpha subunits (CATSPER1–4) and at least eight auxiliary subunits (CATSPERβ, γ, δ, ε, ζ, and θ [
10]; EF-hand calcium binding domain 9; and C2 calcium dependent domain containing 6 [
11]) [
12,
13]. These subunits are conserved between mouse and human, and genetic variations in
CATSPER1,
CATSPER2,
CATSPER3, and
CATSPERE have been found in infertile men [
14,
15,
16]. These results indicate that CatSper is essential for male fertility in mammals.
Ca
2+ is crucial in almost every physiological activity by which sperm acquire their fertilizing capability, including motility, capacitation, hyperactivation, the acrosome reaction, and chemotaxis [
4]. The knockout of mouse
Catsper genes results in male infertility and a lack of CatSper current and hyperactivated motility in sperm [
17,
18,
19,
20]. In humans,
CATSPER1 and
CATSPER2 mutations have been reported to be involved in asthenoteratozoospermia in men. Their sperm lack the CatSper current, accompanied by lower sperm counts and motility [
14,
21]. In particular, A recent study found that the copy number variation of
CATSPER2 causes idiopathic male infertility with normal semen parameters [
15]. The sperm of this patient had very low CATSPER2 protein expression, no CatSper current, and failed to undergo hyperactivation. In addition, a CATSPER-current-deficient infertile man with a homozygous in-frame deletion in
CATSPERE showed normal sperm quality but no hyperactivated motility [
22,
23]. Additionally,
CATSPER3 mutations cause male infertility due to the failure of the acrosome reaction, but there were no defects in routine semen parameters [
16]. Therefore, CatSper plays a central role in the fertilizing capacity of sperm.
CatSper cooperates with other sperm ion channels, exchangers, and transporters to regulate sperm functions in mammals. Sperm-specific Na
+/H
+ exchangers transport H
+ out of the sperm while transporting Na
+ into the sperm plasma membrane, and HV1 expels H
+ from sperm, creating an alkaline environment within sperm [
24]. Correspondingly, the pH-sensitive CatSper is activated, resulting in Ca
2+ influx and the activation of Ca
2+-dependent sperm functions. In addition, in mouse sperm, intracellular alkalinization activates KSper, a sperm-specific potassium channel, which further hyperpolarizes the sperm cellular membrane [
25]. KSper-dependent membrane hyperpolarization increases the force driving Ca
2+ influx through CatSper [
26]. In humans, KSper-induced hyperpolarization further affects CatSper [
26]. Furthermore, Na
+/K
+ ATPase α4 functions as transport to maintain the membrane potential and regulate CatSper directly or indirectly [
24]. The HCO
3− transporters (such as SLC26A3) carry HCO
3− into sperm cells to activate soluble adenylyl cyclase and increase the sperm cyclic adenosine monophosphate (cAMP), which can stimulate CatSper in human and mouse sperm [
24].
After sperm enter the female reproductive tract, the physicochemical and biochemical microenvironment undergo significant changes. As a result, the response of sperm to environmental factors is vital for successful fertilization. CatSper is a polymodal chemosensor in mammalian sperm. It plays a pivotal role in responding to multiple chemical cues including P4, PGs, cyclic nucleotides, ZP glycoproteins, serum albumin, β-defensins (DEFBs), and neurotransmitters, drugs, traditional Chinese medicine, EDCs, and antioxidants. Consequently, understanding the diverse mechanisms by which extracellular factors regulate CatSper is of the utmost importance.
3. CatSper and Physiological Chemicals
In the microenvironment of the female reproductive tract, many physiological chemicals, such as oviducal hormones, regulate sperm functions and increase [Ca
2+]
i [
8]. In these hormones, P4 and PGs are the best known for the oviducal ligands of CatSper. They are secreted by the oviduct and serve as the predominant hormones in follicular fluid [
8]. In addition, several physiological stimuli, including cyclic nucleotides, ZP glycoproteins, serum albumin, DEFBs, neurotransmitters, and odorant attractants, elicit a CatSper-dependent Ca
2+ increase [
27] (Summarized in
Figure 1).
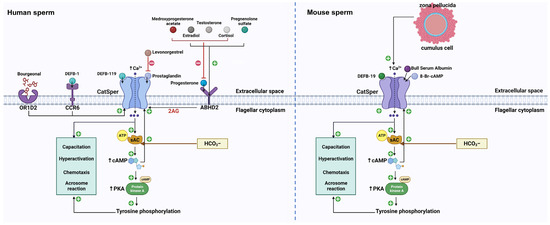
Figure 1. The signaling pathways of different physiological ligands on mammalian CatSper. In human sperm, the P4 binding on the ABDH2 receptor provokes 2-AG depletion and thus removes the inhibition induced by 2-AG on the CatSper channel. Substantially, CatSper opens and allows the extracellular influx of Ca2+ and elevates the concentration of intracellular Ca2+. The activation of sAC triggered by HCO3− and Ca2+ increase the level of cAMP, which causes activation of PKA and tyrosine kinase. cAMP can also activate CatSper channel. As a result, the tyrosine phosphorylation leads to sperm capacitation, hyperactivation, chemotaxis, and acrosome reaction. Apart from P4, pregnenolone sulfate can complete the same ABDH2 binding sites to activate CatSper. Cortisol, testosterone, as well as estradiol, target the same binding sites of P4 to activate CatSper. The P4-induced CatSper activation is suppressed by these three molecules, while the PGE-induced CatSper activation is inhibited by levonorgestrel. Also, medroxyprogesterone acetate exerts an inhibitory effect on P4-induced CatSper activation. Additionally, DEFB-1 binding on the CCR6 receptor can activate CatSper and induce Ca2+ mobilization. In addition, CatSper is also activated by DEBF-19 and bourgeonal, but OR1D2 is involved in bourgeonal-induced CatSper activation. In mouse sperm, DEFB-19, BSA, as well as 8-BR- cAMP, activate the CatSper channel and induce the mobilization of Ca2+ in mouse sperm. Intracellular Ca2+ and HCO3− can activate sAC and elevate the level of cAMP, leading to the activation of protein kinase A and tyrosine kinases. Thus, the tyrosine phosphorylation initiates a certain process related to sperm function, including sperm capacitation, hyperactivation, chemotaxis, and acrosome reaction. In addition, the interaction between ZP and mouse sperm can elevate intracellular Ca2+, which require the CatSper channel to enter mouse sperm. The solid line with the arrow represents activation and the red line represents inhibition. ABDH2: α/β hydrolase domain containing protein 2, 2-AG: 2-arachidonoylglycerol, CCR6: C-C chemokine receptor, DEFB-1: β-defensins 1, DEFB-119: β-defensins 119, DEFB-19: β-defensins 19, OR1D2: olfactory receptor, sAC: soluble adenylyl cyclase, PKA: protein kinase A, BSA: bull serum albumin, ZP: zona pellucida.
3.1. CatSper and Endogenous Steroids
As sperm travel through the female reproductive tract, they are exposed to a variety of steroid hormones. Human follicular fluid (HFF) present in the female reproductive tract is a key factor for human fertilization; it is present at every stage of impregnation. P4, secreted by the oviductal epithelium and cumulus cells, is the predominant hormone in HFF [
8]. P4 can elevate [Ca
2+]
i of mammalian sperm [
28]. However, the activation of CatSper by P4 has only been reported in humans and rhesus macaques [
29]. In murine sperm, P4 cannot activate mouse CatSper, although it increases sperm [Ca
2+]
i [
30]. Therefore, CatSper regulation likely occurs via species-specific mechanisms. Under normal physiological conditions, endogenous P4 activates human CatSper through non-genomic actions mediated by the P4/abhydrolase domain containing the 2 (ABHD2)/CatSper/Ca
2+ axis. This pathway relies on the coordinated action of ABHD2, a P4 receptor expressed in sperm, and endocannabinoid 2-arachidonoylglycerol (2-AG), an endogenous CatSper inhibitor [
31]. P4 binds to ABHD2 and triggers the depletion of 2-AG within the sperm plasma membrane and the release of CatSper from 2-AG inhibition, allowing Ca
2+ influx [
31]. P4-induced Ca
2+ influx triggers multiple Ca
2+-dependent physiological responses, including hyperactivation, the acrosome reaction, and chemotaxis, which are critical for successful fertilization [
30,
32].
Another P4-like steroid hormone, pregnenolone sulfate, competes with P4 for the same ADBH2 binding site to activate CatSper in humans [
33,
34]. In addition, several endogenous steroids, such as testosterone, cortisol, and estradiol, have been identified as CatSper agonists [
34]. Interestingly, in human sperm, these steroids target the same P4 binding site to activate CatSper, and they dose-dependently inhibit CatSper-dependent Ca
2+ influx induced by P4 [
35]. A recent study demonstrated that high cortisol levels in human sperm inhibit the P4-induced Ca
2+ response. The presence of high cortisol levels resulting from anxiety symptoms exerts a competitive inhibitory effect on P4-induced Ca
2+ influx and the acrosome reaction, ultimately compromising the quality of semen and fertility potential [
36]. This suggests that cortisol, a potential stress biomarker, could negatively impact male reproductive health. Interestingly, certain steroids exhibit inhibitory effects on ligand-induced CatSper activation in a selective manner in human sperm [
37]. Unlike mibefradil, which inhibits Ca
2+ influx induced by all steroid hormones, medroxyprogesterone acetate, levonorgestrel, and aldosterone selectively suppress CatSper-dependent Ca
2+ influx induced by P4, PGs, and the fungal pheromone sirenin in human sperm [
37].
3.2. CatSper and PGs
Besides P4, PGs are also oviducal ligands for CatSper in human sperm. They are secreted by the oviduct, and the cumulus cells surrounding the oocyte are important ligands for CatSper. In HFF, the coexistence of P4 and PGs could elevate [Ca
2+]
i. In addition, seminal fluids contain high concentrations of PGs [
38]. In the epididymis, sperm gain their fertilizing ability and maturity, and PGs may affect sperm function during this period. CatSper activation by PGs at the correct time is critical for successful fertilization. During the ejaculatory process, Zn
2+ in seminal fluid inhibits PG-induced Ca
2+ influx of human sperm, thereby preventing premature activation of CatSper and facilitating sperm escape into the female genital tract to localize the egg that is ready for fertilization [
8]. Prostaglandin E1 (PGE1) activates CatSper, increases [Ca
2+]
I in a biphasic manner with similar amplitudes, and potentiates Ca
2+ currents similarly to P4 in human sperm [
39]. Consistently, PGs do not activate mouse sperm CatSper [
30]. This emphasizes the differential regulation of CatSper between humans and mice. Interestingly, there is synergistic activation of human CatSper when PGE-1 and P4 are combined. This result indicates that PGE1 and P4 activate CatSper, apparently through two different binding sites or signaling mechanisms [
38,
39]. In addition, PGs activate human CatSper with different potencies: PGE1 > PGA1 > PGE2 ≫ PGD2 [
30]. However, the mechanism of action of PGs on CatSper has yet to be fully elucidated [
9,
39].
3.3. CatSper and cAMP
The cAMP is a very important physiological chemical and plays a vital role in signaling transduction. In mammalian sperm, the cAMP pathway is essential for sperm functions, such as capacitation and hyperactivation. A study showed that 8-Br-cAMP, an analog of cAMP, increases CatSper-dependent [Ca
2+]
i [
40] and modulates P4 to ultimately increase [Ca
2+]
i in mouse sperm [
41]. In addition, bicarbonate can activate soluble adenylate cyclase, increase cAMP levels, and stimulate CatSper in human and mouse sperm [
42,
43,
44].
3.4. CatSper and ZP Glycoproteins
The ZP acts as a protective matrix surrounding the oocyte in the female reproductive tract. In mammalian fertilization, the interaction between sperm and ZP glycoproteins triggers an increase in sperm [Ca
2+]
i [
45]. Xia and Ren [
45] found that ZP glycoproteins trigger Ca
2+ entry into mouse sperm via CatSper. Balbach et al. [
46] showed that ZP glycoproteins evoke a rapid increase in intracellular pH, and CatSper translates this change into a Ca
2+ response in mouse sperm. In addition, sperm from
Catsper1 knockout mice do not exhibit the ZP-glycoprotein-induced [Ca
2+]
I elevation. Indeed, the Ca
2+ mobilized by ZP glycoproteins requires CatSper to enter sperm, implying that ZP-glycoprotein-induced Ca
2+ influx is dependent on CatSper [
45,
47]. The knockout of
Catsper genes in mice diminishes the ZP penetration and sperm motility. Thus, CatSper is necessary for sperm to penetrate the ZP effectively.
3.5. CatSper and Bovine Serum Albumin (BSA)
Capacitation is a functional maturation process that is necessary to produce hyperactivated motile sperm [
5]. This process is dependent on extracellular Ca
2+. BSA can induce sperm capacitation and increase [Ca
2+]
i in several mammals, but these effects are absent in the sperm of
Catsper1-knockout mice and could be restored by an EGFP-CATSPER1 fusion protein [
48]. These results suggest that BSA promotes Ca
2+ entry into sperm via CatSper.
3.6. CatSper and DEFBs
The DEFB family includes small antimicrobial peptides expressed in the reproductive tract and involved in sperm motility and fertilization [
49]. DEFB1 was the first identified member of the DEFB family; it is secreted by the epithelium of the male genital tract [
50]. In human sperm, DEFB1 binds to its sperm receptor, C-C chemokine receptor 6, and evokes CatSper-dependent Ca
2+ flux to regulate sperm motility, hyperactivation, and the acrosome reaction [
51]. In addition, DEFB19/119 (mouse/human orthologs), secreted by the female germinal duct epithelium and the oocyte-ovarian complex, elicits Ca
2+ mobilization via CatSper and induces sperm chemotaxis in capacitated sperm [
52]. Mouse DEFB19 and human DEFB119 can activate the CatSper current in mouse and human sperm, respectively [
52].
Defb19 knockdown in mouse oviducts and
Defb19 knockout in male mice impairs sperm chemotaxis. In humans, DEFB119 expression and chemotactic activity are markedly decreased in HFF collected from women with idiopathic infertility [
52]. These results indicate that DEFB19/DEFB119 play important roles in sperm chemotaxis and are associated with idiopathic infertility.
3.7. CatSper and Neurotransmitters
In mammals, receptors for many neurotransmitters and neuromodulators (such as acetylcholine, adenosine, adenosine triphosphate, γ-aminobutyric acid, serotonin, norepinephrine, and dopamine) are found in sperm. Therefore, a sperm is regarded as a neuron with a tail [
53,
54]. Interestingly, P4 activates CatSper in human sperm via an unconventional endocannabinoid signaling pathway (P4/ABHD2/2-AG/CatSper) [
31]. In addition, serotonergic signals enhance hamster sperm hyperactivation via CatSper [
55].
3.8. CatSper and Odorant Attractants
Sperm chemotaxis guides sperm toward the oocyte and is closely related to sperm capacitation, hyperactivation, the acrosome reaction, and male fertility. In humans, bourgeonal is a typical odorant and chemoattractant that is proposed to activate olfactory receptors (OR1D2) and to open CatSper to increase [Ca
2+]
i via a G-protein-coupled receptor/olfactory G-protein/cAMP/PKA pathway [
9,
56,
57]. Moreover, men with idiopathic infertility and low sensitivity to bourgeonal have decreased OR1D2 protein expression and bourgeonal-activated CatSper current in their sperm [
58]. These findings link odor perception to CatSper and male infertility. This sperm odorant attractant may provide a feasible screening method for CatSper-related male infertility.
4. CatSper and Medicines
4.1. CatSper and Traditional Medicine
CatSper is regarded as a primary target for the pharmacological treatment of male infertility and a novel non-hormone target for male contraception. Some traditional medicine has shown promise for improving male infertility through CatSper. Sheng Jing Shan (SJS), a traditional Chinese medicine, has shown efficacy in treating asthenozoospermia. Notably, SJS effectively improved the sperm motility of a rat model of cyclophosphamide (CP)-induced asthenozoospermia by upregulating
Catsper1 expression [
59]. Trigonelline semen (TS), also known as fenugreek, is a natural herbal substance recognized for its ability to improve sperm count and motility in infertile men [
60]. In a rat model of CP-induced male infertility, TS effectively restored sperm count, motility, testosterone levels, and the expression of
Catsper1,
Catsper2,
Catsper3, and
Catsper4 [
61].
Panax ginseng, a well-known traditional medicine with multiple pharmacological activities, is beneficial in treating various diseases [
62]. Regarding male fertility, studies have noted that mice treated with
P. ginseng exhibit increased sperm motility and Ca
2+ levels [
63].
P. ginseng significantly increases the expression of
Catsper1,
Catsper2,
Catsper3, and
Catsper4 in mouse sperm [
63]. A recent investigation reported that a natural herb called
Putranjiva roxburghii could effectively upregulate the expression of CatSper genes in bull sperm and markedly boost sperm motility [
64]. In addition, escanbil is a traditional medicine applied to treat abnormal menstruation and menstrual cramps in folk medicine [
65]. It improves sperm motility and alters the expression of CatSper genes in aging mice [
66]. These results suggest that CatSper may be a potential therapeutic agent for natural medicine treatment of male infertility.
4.2. CatSper and Anti-Depressants
Selective serotonin reuptake inhibitors are the most widely used antidepressants in the United States and Europe, but recent research has highlighted their potential to impair male fertility [
71]. Researchers have demonstrated that sertraline inhibits CatSper currents induced by intracellular alkalinization, voltage changes, P4, and PGs in human sperm [
72]. Sertraline has a significant inhibitory effect on the acrosome reaction and viscous-medium penetration induced by P4 and PGs [
72]. These findings suggest that the therapeutic administration of sertraline for depression may impair human reproduction.
4.3. CatSper and 5-Alpha Reductase Inhibitors
Finasteride (FS) and dutasteride (DS), two 5-alpha reductase inhibitors, are widely used to treat benign prostate hyperplasia. However, their prolonged use has been shown to adversely affect male semen quality [
73]. FS activates CatSper, at least partially, via PG binding sites, whereas DS activates CatSper, at least partially, through P4 binding sites in human sperm [
74]. Thus, they interfere with Ca
2+ signaling mediated by PGs and P4. In addition, the exposure of mice to DS affected sperm count and motility and the expression of CatSper genes in caput and caudal epididymal sperm [
75].
4.4. CatSper and Analgesics
Paracetamol is widely used as a mild analgesic to alleviate fever and pain. However, rodent studies have shown that paracetamol may have negative effects on sperm count and motility due to its endocrine effects. Additionally, high concentrations of paracetamol in male urine have been linked to lower sperm motility [
76]. In human sperm, paracetamol is metabolized to N-arachidonoylphenolamine via fatty acid amide hydrolase expressed in the sperm neck region. N-arachidonoylphenolamine directly activates human CatSper, reduces sperm motility, and affects viscous-medium penetration [
77].
4.5. CatSper and Ca2+ Channel Blockers
Nifedipine is a Ca
2+ channel blocker and is used as an antihypertensive medicine. It exhibits anti-fertility effects in male rats, resulting in a significant reduction in sperm motility and count [
78]. Nifedipine treatment reduces sperm motility and count and substantially downregulates the expression of CatSper genes in mouse epididymal sperm [
75]. In addition, RU1968, a steroid-based selective and potent cross-species inhibitor of CatSper, has been demonstrated to suppress the activation of CatSper in human, mouse, and sea urchin sperm [
79]. Therefore, nifedipine serves as a powerful tool for the investigation of the physiological function of CatSper in human sperm and for the promotion of the development of non-hormonal male contraceptives.
4.6. CatSper and Phosphodiesterase (PDE)-Inhibitors
Trequinsin hydrochloride, a PDE-3 inhibitor, has emerged as a promising CatSper agonist. In human sperm, trequinsin hydrochloride exhibits a P4-like agonist profile and significantly potentiates the CatSper current, effectively increasing sperm hyperactivation and viscous-medium penetration [
80]. Additionally, this CatSper agonist induces a concentration-dependent elevation in Ca
2+ levels through cross-desensitization with PGE1 [
80].
4.7. CatSper and Anti-Inflammatory Drugs
Cisplatin is the most widely used drug in oncology treatment. However, cisplatin-based treatment of testicular cancer disrupts spermatogenesis and reduces the sperm motility of patients [
81]. The indole derivative N′-(4-dimethylaminobenzylidene)-2-1-(4-(methylsulfinyl) benzylidene)-5-fluoro-2-methyl-1H-inden-3-yl) acetohydrazide (MMINA) has significant anti-inflammatory and antioxidant effects and can protect against the testicular toxicity induced by cisplatin [
82]. Most importantly, MMINA activates CatSper by upregulating the expression of CatSper genes in rat sperm [
83]. Moreover, MMINA is capable of forming hydrogen bonds with CatSper [
83].
5. CatSper and EDCs
5.1. CatSper and Environmental Estrogens
Initially, EDCs were called xenoestrogens due to their estrogenic, antiestrogenic, androgenic, and antiandrogenic effects [
85]. Steviol, a natural non-caloric sweetener metabolite, exerts endocrine effects on human sperm by antagonizing P4 and agonizing CatSper, resulting in a rapid influx of Ca
2+ [
86]. Bisphenol A (BPA), a ubiquitous EDC and synthetic organic compound, has been significantly and negatively associated with male fertility [
87]. BPA binds to estrogen receptors α and β and exhibits estrogenic activity [
88]. Animal studies have revealed that BPA impairs sperm function by reducing the expression of CatSper genes and the CatSper current [
89]. In GC-2 cells, a mouse spermatogonia cell line, BPA decreased the growth rate and [Ca
2+]
i, and downregulated the expression of
Catsper1,
Catsper2,
Catsper3, and
Catsper4 through Ten-eleven translocation 1 [
90]. In humans, bisphenol A diglycidyl ether and bisphenol analogs—but not BPA—activate CatSper [
91]. Diethylstilbestrol, a well-known, synthetic, non-steroidal estrogen, potentiates CatSper currents, increases the [Ca
2+]
i, and inhibits P4-induced Ca
2+ influx and sperm functions in humans [
92]. Perfluorooctane acid, an organic pollutant, activates CatSper to elevate the [Ca
2+]
i in human sperm [
93]. Like diethylstilbestrol, perfluorooctane acid suppresses the P4-induced CatSper current, Ca
2+ influx, and sperm functions [
93]. In addition, the diversity of EDCs implies that even heavy metals may possess estrogenic activity. Cadmium is considered an EDC with significant toxicity to the reproductive system; it acts as an estrogen mimic and has the ability to bind ERs [
94]. Cadmium impairs sperm function via a CatSper-mediated mechanism by affecting the expression of CatSper genes in mice [
95].
5.2. CatSper and Pesticides
p,p′-Dichlorodiphenyldichloroethylene, a metabolite of dichloro-diphenyl-trichloroethane commonly found in human reproductive fluids, activates CatSper to induce Ca
2+ entry into sperm and disrupts acrosome reaction [
96]. Pentachlorophenol, a widely used pesticide, suppresses the P4-induced CatSper current, Ca
2+ influx, and sperm functions in humans [
97]. Recently, a study investigated the effect of 53 pesticides and pesticide metabolites on human sperm. The results demonstrated that, although 26 pesticides activated CatSper and interfered with signaling triggered by P4 and PGs, they may interact with the unique binding sites or the P4 and PG binding sites of CatSper [
98]. Thus, pesticides, either alone or in low-dose mixtures, have the potential to negatively affect sperm function by interfering with normal Ca
2+ signaling in human sperm via CatSper.
5.3. CatSper and Chemical Ultraviolet (UV) Filters
Chemical UV filters, commonly present in daily-use sunscreens, are among the most potent triggers of Ca
2+ signaling. They directly activate CatSper in human sperm and elevate [Ca
2+]
i [
84]. A recent study investigated the effect of 31 chemical UV filters approved in the European Union and the United States on human sperm. Although 29 of the 31 chemical UV filters induced Ca
2+ signaling in human sperm, only nine of these chemicals could activate CatSper, including 4-Methylbenzylidene camphor, 3-Benzylidene camphor, meradimate, amiloxate, octisalate, benzylidene camphor sulfonic acid, homosalate, benzophenone-3, and octinoxate [
99]. Of these chemicals, 3-Benzylidene camphor, benzylidene camphor sulfonic acid, and 4-Methylbenzylidene camphor have been found to competitively inhibit P4-induced Ca
2+ signaling and target its binding sites in CatSper [
84,
99]. These results suggest that some chemical UV filters have the potential to interfere with P4-induced Ca
2+ signaling and negatively affect sperm functions.
6. CatSper and Drugs of Abuse
Interestingly, some addictive drugs affect sperm functions through CatSper. Methamphetamine (METH) is a highly addictive central nervous system stimulant that has detrimental effects on male reproductive health, including impaired spermatogenesis, testicular damage, and abnormal sperm quality [
100]. In particular, a novel investigation showed that rats receiving METH resulted in a decrease in testis and epididymis weight [
101]. Meanwhile, the relative expression levels of
Catsper1,
Catsper2,
Catsper3, and
Catsper4, as well as the sperm motility associated gene
Mvh, were decreased significantly [
101]. In addition, the exclusive expression of
Catsper1–4 in testes is required for sperm motility and fertility [
21,
102]. As a result, the downregulation of these genes induced by METH increases the possibility of male infertility. Therefore, men addicted to METH may encounter potential reproductive problems.
Ketamine, a dissociative anesthetic widely used in human and animal medicine, has become a popular recreational drug because it can induce hallucinatory effects. ketamine affects sperm motility, viscous-medium penetration, and the P4-induced acrosome reaction by inhibiting CatSper in human sperm, thus decreasing [Ca
2+]
i [
103]. In addition, ketamine is an antagonist of the N-Methyl-D-aspartic acid (NMDA) receptor. The NMDA receptor is expressed in human sperm and involved in the inhibitory effect of ketamine on human sperm functions [
104]. Specifically, NMDA, the physiological ligand of NMDA, could partly alleviate the motility of human sperm and significantly recover the capacitation and acrosome reaction, as well as [Ca
2+]
i [
104]. Therefore, the competitive receptor binding between ketamine and NMDA may provide novel insight for clinical diagnoses of ketamine abusers. Collectively, CatSper-related drugs of abuse have been implicated in impaired sperm function and/or male infertility.
7. CatSper and Antioxidants
Oxidative stress occurs when the generation of reactive oxygen species (ROS) exceeds the natural antioxidant defenses of bodies. Thus, the precise balance of ROS and antioxidants within sperm are necessary for capacitation and fertilization. The major effect of oxidative stress compromising sperm function is caused by two principal mechanisms, DNA damage and lipid peroxidation [
105]. In human sperm, ROS damages DNA directly by the production of 1,N
6-ethenoadenosine and 1,N
2-ethenoguanosine, resulting in DNA structure instability and leading to single-strand breaks [
106]. Once the transcription and translation of post-spermiogenesis stop, the DNA repair during developing sperm is terminated [
107]. Hence, sperm function and pregnancy outcome are strongly impacted.
To counteract ROS damage, the human body has developed a variety of antioxidant strategies. For instance, non-enzymatic antioxidants contained within the seminal fluid, like vitamin E and selenium [
108]. Interestingly, treatment with these two antioxidants upregulates the expression of
Catsper in the testes of young adult and aged male mice, which are the genes responsible for sperm motility [
109,
110]. Meanwhile, sperm parameters such as viability rate and morphology also show an improvement after treatment [
109,
110]. Consequently, these two essential components play a crucial role in the maintenance of male reproduction.