Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Parasitology
Giardia duodenalis (syn. G. lamblia, G. intestinalis) is an intestinal protozoan parasite that causes giardiasis in humans and livestock, companion and wild animals. Giardiasis is a leading cause of waterborne diarrheal infections worldwide, with an estimated number of 280 million human cases per annum.
- extracellular proteases
- cysteine proteases
- papain-like proteases
1. Introduction
Protistan parasites of the genera Giardia, Entamoeba, Cryptosporidium and Blastocystis cause intestinal infections and diseases (giardiasis, amoebiasis, cryptosporidiosis and blastocystosis) in humans and animals worldwide. Current evidence indicates that these pathogens collectively affect >1.5 billion people and cause ~300,000 deaths per annum [1,2,3]. These figures are likely to get worse due to climate change, extensive human travel and increased globalization.
All of these parasites colonize the intestinal tract and can cause acute or chronic diarrhea, malabsorption and/or bleeding, with severe sequelae, such as chronic bowel inflammation, reduced growth and development and in severe cases, death. However, infections can be self-limiting or asymptomatic, reflecting a degree of adaptation of these pathogens to their hosts and/or the effectiveness of host immune responses to suppress infections [4]. These pathogens have distinctive life cycles, which are linked to their particular tropism. For instance, Blastocystis and Giardia are extracellular parasites and Cryptosporidium is intracellular/extra cytoplasmic, whereas E. histolytica can invade tissues from the large intestine to spread (via the bloodstream) to the liver, spleen, kidney, brain and other organs, enabled by cellular structures for locomotion, attachment and penetration as well as by molecules acting on intra-, epi- or extracellular levels to promote parasite establishment and survival within the host.
Parasite structures and/or molecules that have an adverse effect on host intestinal homeostasis through mechanical, metabolic and functional damage can be virulence factors. These can include surface-anchored or secreted molecules such as toxins, lectins, cysteine-rich proteins, lytic enzymes (proteases, lipases and nucleases), metabolic enzymes and molecules with multiple functions, which are often called “moonlighting” proteins [5,6,7,8]. In the intestinal lumen and in intraepithelial compartments, intestinal protozoa and the molecular products that they release must overcome multiple barriers, such as mucus, epithelial cell surfaces (e.g., via microvilli, receptors and/or channels), intercellular junctions (tight and/or adherent junctions and desmosomes) and elements of the innate and adaptive immune systems (phagocytic/cytotoxic cells, IgA, antimicrobial peptides and cytokines). In this context, peptide hydrolases (proteases) play a key role. Seven broad groups of hydrolytic (i.e., aspartic, asparagine, cysteine, glutamic, metallo-, serine and threonine) proteases are classified in the current release (12.4) of the MEROPS database (https://www.ebi.ac.uk/merops/cgi-bin/family_index?type=P; accessed date: 2 August 2023) [9]. Cysteine proteases (CPs) are the most diverse type (families C1–C124), and tens to hundreds of CPs are encoded in the genomes of intestinal protists.
Several CPs are synthesized as inactive zymogens, which, upon proteolytic removal of an N-terminal prodomain, become catalytically competent. The catalytic mechanism of CPs involves the following steps: (a) deprotonation of a cysteine at the active site by an adjacent proton acceptor (usually histidine) that renders a thiolate-imidazolium charge, which is stabilized by a neighbor asparagine; (b) nucleophilic attack at the carbonyl carbon by the cysteine thiolate (proton acceptor), which promotes the release of the first half of the product and a thioester intermediate; (c) hydrolysis of the acyl-enzyme intermediate by activated water, which, in turn, releases the second half of the product with a terminal carboxylic acid, converting a CP into its initial state [10]. According to the MEROPS protease database, there are 14 CP groups called “clans” including nine C-type and five P-type groups. Evidence suggests that CPs of intestinal protozoa emerged from convergent evolution of the catalytic mechanism characteristic of clan CA (papain-like); in this clan, the most studied, abundant and relevant CPs are those in families C1 (cathepsin B/L-like) and C2 (calpain-like) [11,12].
2. Cysteine Proteases Secreted by G. duodenalis: An Arsenal of Cathepsin-like Enzymes with Multiple Targets
Giardia duodenalis (syn. G. lamblia, G. intestinalis) is an intestinal protozoan parasite that causes giardiasis in humans and livestock, companion and wild animals. Giardiasis is a leading cause of waterborne diarrheal infections worldwide, with an estimated number of 280 million human cases per annum. Giardiasis is included in the WHO’s Neglected Disease Initiative [13], and Giardia is considered a potential bioterrorism agent (category C) [14]. During its life cycle, Giardia alternates between the vegetative, pathogenic trophozoite and dormant, infective cyst forms. When a susceptible host ingests cysts contaminating water and food, excystation occurs during passage through the stomach into the small intestine. Emerging excyzoites rapidly divide by binary fission to give rise to trophozoites, which adhere to enterocytes via their adhesive disks and proliferate and progressively colonize the epithelial surfaces of the duodenum and midjejunum. Here, trophozoites are exposed to an alkaline pH and biliary secretions; cholesterol deprivation induces encystation, leading to the formation of cysts, which are shed in the feces.
Giardia infection can result in disease (giardiasis), which is symptomatic (acute to chronic) but can also be asymptomatic. The pathogenesis of giardiasis relates to virulence factors, which become active at the interface between trophozoites and small intestinal epithelium and are produced/released by trophozoites. These factors include cathepsin B proteases, tenascins, cystatin and a number of variant-specific surface proteins (VSPs) [15]. Of the secreted products, CP activity accounts for almost all of the parasite-derived proteolysis from supernatants recovered from trophozoite–epithelial cell interaction assays [16].
It has been reported that the genome of G. duodenalis (reference clone WBA6) encodes 25 CPs. Of these, 21 are cathepsin type: 8 cathepsin-L, 9 cathepsin-B with endopeptidase activity, 3 cathepsin-B with endopeptidase and dipeptidyl peptidase activity (associated with the presence of an ‘occluding loop’) and 1 cathepsin-C-like (the encystation-specific CP, ESCP) [17]. The repertoire of the cathepsin-like gene varies among strains and assemblages. Thus, in assemblage B, the GS strain has 17 homologs, while strain GS-B has 23; in assemblage E, the P15 strain contains 23 homologs. These proteases are activated following proteolytic removal of the N-terminal prodomain of their inactive precursors. During trophozoite growth and encystation, differential expression of cathepsin-B-like proteases occurs, including those formerly named CP2, CP4 and CP5, as well as the cathepsin-L-like protease CP17 [18], which are abundantly expressed [19]. This finding suggests that this family of proteases has diverse functional roles, depending on their location in the cellular compartments. These proteases, which are expressed and released by the parasite, have gelatinolytic and/or collagenolytic activities [16,20].
Interestingly, in G. duodenalis, some CPs are also encoded by other genes representing >230 variant surface proteins (VSPs). These VSPs are expressed as glycoproteins and/or palmitoyl-proteins, and their genes are transcriptionally regulated by an iRNA-silencing mechanism (named “all but one”) and are also involved in antigenic variation and the induction of protective immune responses. In this family, there is at least one clade of CPs with structural and/or functional similarities to cathepsins B. One of them is VSP9B10A (739 amino acids (aa) long; GL50803_101074), which can be detected in a secreted form (~75 kDa) during trophozoite–epithelial cell monolayer (IEC-6) interactions and displays proteolytic activity [21]. VSP9B10A contains a central region (aa 324 to 684) with a catalytic substrate binding pocket and calcium recognition sites of a typical cathepsin B. This atypical cathepsin is not always expressed on the trophozoite surface but has been shown to be toxic to epithelial monolayers [21], leading to a loss of cell-cell and cell-substrate contacts (Figure 1). Experimental evidence has demonstrated that VSP9B10A is a conditional virulence factor [21].
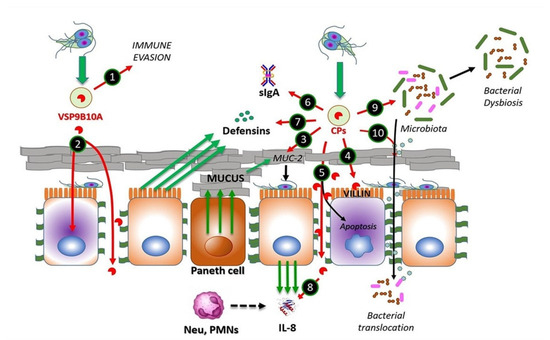
Figure 1. CPs from Giardia duodenalis trophozoites interact with multiple targets in the small intestine. Experimental models of trophozoites–epithelial cell interactions indicate that the parasite releases CPs, mainly cathepsin B-type, including Giardipain-1 (GL50803_14019), GL16160 and GL16779 and non-canonical CPs (e.g., VSP9B10A). This latter CP may serve, when expressed, to divert the immune system (1) or may cause damage to the epithelium due to a loss of cell–cell junction and cytotoxicity in the cell (2). Secreted CPs have been shown to degrade substrates, such as mucin 2, an important component of intestinal mucus (3), enabling trophozoite adhesion to microvilli. Direct damage to epithelial cell integrity by CPs may include disruption of the cytoskeletal microvillus-resident protein villin (4) and the disruption of tight junction proteins, such as ZO-1 and claudins, also involving adherens junction proteins including β-catenin or E-cadherin (5). Soluble elements of the innate immune response, including secretory IgA (6), produced by plasmatic B-cells, defensins (7) and IL-8 (8), both produced by epithelial cells, of which the latter works as neutrophil attractant, might be degraded by giardial CPs. These enzymes also provoke alterations in the microbiome of the small intestine, leading to dysbiosis (9), while bacterial translocation from the luminal to the intraepithelial compartment (10) may be promoted via the degradation of intercellular junctions by CPs as mentioned. Recent studies suggest that giardial CPs might be secreted after removal of N-terminal prodomain and inclusion into membrane-bound extracellular vesicles (EVs), mainly exosomes (green-filled circles).
Giardia CPs might be regulated at the intracellular level by cystatins, as is the case for Cryptosporidium (cryptostatin) [22]. Cystatins comprise a superfamily of proteins with a homologous sequence and structure, consisting of an antiparallel β-sheet topped with an α-helix. These function as endogenous cysteine protease inhibitors of target proteases, such as cathepsins B, C, L and H, and are currently classified into type 1 (stefins), type 2 (cystatins), type 3 (kininogens), latexins and fetuins [23]. In G. duodenalis, a stefin (GL50803_27918) was identified as a cytoplasmic protein that potently inhibits three major secreted CPs of the clan CA papain-type, namely GL50803_14019 (Giardipain-1), _16160 and _16779. Interestingly, stefin is a weak inhibitor of human cathepsin B [24] and might play a role in Giardia–epithelial cell interaction, as previous evidence revealed an upregulation of cathepsin-L-like proteins GL50803_137680 or GL_50803_3099 [25] upon the interaction of distinct strains (WB, P-1, GS/M or NF) of Giardia with ICE-6 monolayers [26]. Another interaction study [27] revealed conspicuous expression of cathepsin B-like proteases in the WB strain of Giardia (i.e., GL50803_16160, _16468 and _16779) and their homologs in the GS strain (i.e., GL50581_2946, _438 and _78), in addition to other cathepsins B (including GL50803_14019 and GL50581_2036). A proteomic study of the same strains (using the same intestinal cell line) reported a distinct repertoire of three cathepsin B-like proteases in the WB strain of Giardia (i.e., GL50803_15564, _16468 and _17516) and their closest homolog in the GS strain (GL50581_2036, _438 and _2318) [28]. This information suggests that trophozoites of the zoonotic assemblages A and B (represented by the strains WB and GS, respectively) exhibit similar CP secretion profiles upon interaction with epithelial cells.
Following secretion, these proteases become catalytically competent due to the absence of an occluding loop and the intracellular excision of prodomains. In the case of Giardipain-1 (GL50803_ 14019, formerly named CP2), its precursor (33 kDa) is 8 kDa larger, i.e., 76 amino acids (aa) longer than the mature protein (25 kDa), which possesses gelatinolytic activity and exhibits pro-apoptotic effects on IEC-6 and epithelial cell (MDCK) monolayers [29]. The CPs secreted by Giardia have different protein targets on host epithelial cells and eventually disrupt intestinal homeostasis, in turn causing small intestinal damage [30] (cf. Figure 1).
The mechanism underlying the secretion of Giardia CPs is not yet fully understood. However, there is evidence for the processing of the N-terminal prodomain of clan CA proteases, such as Giardipain-1 [18,29]. In a recent proteomic study of extracellular vesicles (EVs; <100 nm in size) released by Giardia trophozoites, cathepsin B-like proteases have been identified as cargo within exosomes [31] (Figure 1). Considering the role of EVs in the intercellular crosstalk between parasites and intestinal epithelial cells, it is likely that these vesicles may mediate the release of cargo proteins around or within epithelial cells to promote cellular and tissue damage.
One of the first barriers that trophozoites encounter is the mucus layer on the brush border of the intestinal epithelium. This layer is mainly composed of membrane-bound mucins, such as MUC4 and MUC16, and gel-forming mucins, such as MUC2, the latter of which predominates in the gastrointestinal tract [32]. Interestingly, the use of a human mucus-producing colonic cell line (LS174T) in interaction assays using excretory/secretory proteins (ESPs) from strains NF (assemblage A) and GS/M (assemblage B) revealed a concentration-dependent degradation of MUC2, which was abrogated by pre-treatment with a CP inhibitor, called E-64 [33] (Figure 1). In addition, a recent investigation [34] reported a novel mechanism by which Giardia CPs modulate goblet cell activity via the cleavage and activation of protease-activated receptor 2 (PAR-2), which can regulate mucin gene expression in intestinal goblet cells.
Another barrier against Giardia is the brush border at the apical surface of enterocytes. However, it has been shown that trophozoites adhering to the microvillus lining of the enterocytes cause damage to the intestinal epithelium, leading to structural alterations and decreased absorptive surface area [35]. In this context, villin, an actin-binding protein expressed in the microvillus layer of enterocytes, can be degraded and redistributed by the action of surface-exposed Giardia CPs (Figure 1), supported by evidence showing the intact villin structure of Caco-2 cells exposed to trophozoites pre-incubated with inhibitors, such as the membrane-permeant E-64 or ML-9 (inhibitor of myosin light chain phosphorylation, MLCK) [36].
Further damage by proteases secreted by G. duodenalis trophozoites can also occur between cells in the intestinal epithelium. This has been shown via the disruption of transmembrane, plaque and filamentous proteins intervening apical junctional complexes (AJCs), known as tight junctions (claudins, occludins and/or zonula occludens—ZO-proteins), and adherent junctions (E-cadherin and β-catenin) [29,37]. There is clear evidence that Giardia ESPs, when added to Caco-2 cells, can alter the distribution of AJC components, such as claudin-1, occludin, ZO-1 and intracellular F-actin [37]. Furthermore, when added to IEC-6 monolayers, purified secreted protease Giardipain-1 has been shown to colocalize with claudin-1 and occludin at cell junctions, affecting distribution, decreasing transepithelial electrical resistance and inducing apoptosis, leading to pathological changes in epithelial cells [29]. Other experiments have shown that recombinant forms of each of the three secreted CPs, designated GL50803_14019 (Giardipain-1), GL50803_16160 and GL50803_16779 (formerly CP3) [27], affect the distribution of AJC proteins [37] and also degrade E-cadherin and occludin, which could be reduced by inhibitor E-64d [37]. Further, the three proteases degraded claudin-1 and -4 and β-catenin, respectively, suggesting that the limited number of CPs released by trophozoites have a redundant activity on cell junction proteins (Figure 1). The proteolytic activity of secreted proteases on specific substrates was shown using recombinant proteins in a two-thioredoxin system. Activity was associated with Giardipain-1, GL50803_16160 and GL50803_16779. Similar substrate selectivities were observed for Giardipain-1 and GL50803_16160; however, these differed from GL50803_16779 [24,38].
Bioinformatics analyses performed to screen the human small intestinal proteome to determine the cleavage consensus sequence of GL50803_16779 predicted immunoglobulins (Ig), defensins (Df) and chemokines (Ck) as targets, suggesting that parasite proteases interfere with adaptive host immune responses (Figure 1) [24,37,38,39,40]. Moreover, the different proteases degraded recombinant human defensins αDf1, βDf5 and αDf6, whereas the activity of Giardipain-1 was higher than those recorded for other proteases. Giardipain-1 may participate in neutralizing the innate response induced by Paneth cell defensins [24,38]. Chemokines/cytokines produced by epithelial cells, including CXCL1–3, are upregulated upon exposure to trophozoites [39]. In this context, it has been shown that interleukin-8 (CXCL8) is degraded by Giardia CPs, thereby reducing neutrophil recruitment and inflammatory responses [40], while recombinant (and upregulated) chemokines/cytokines CXCL1–3, CXCL8, CCL2 and CCL20 were cleaved by either ESPs or recombinant GL-50803_16160 [37]. These studies suggest that CPs participate in the onset and establishment of Giardia infection and also modulate host immune responses.
In experiments in which device-isolated biofilms and microbiota-free mice were used, it was observed that secreted CPs play a role in the interplay of Giardia with the commensal microbiota communities located in the gut as biofilm-forming, planktonic bacteria. In these studies, a proinflammatory response was observed, even in the absence of Giardia, suggesting that CPs from Giardia play a role in intestinal disease, including irritable bowel ensuing infection [41,42]. Interestingly, recent experiments from researchers' group [30] have shown that, in an experimental infection system, purified Giardipain-1 induces apoptosis and the extrusion of epithelial cells at the tips of the villi in ligated intestinal loops in jirds (Meriones unguiculatus). Moreover, the infection of jirds with trophozoites expressing Giardipain-1 resulted in intestinal epithelial damage, cellular infiltration, crypt hyperplasia, goblet cell hypertrophy and edema. Pathological alterations were pronounced when jirds were infected (via gavage) with Giardia trophozoites that stably overexpressed Giardipain-1. Furthermore, Giardia colonization in jirds resulted in chronic inflammation, which might relate to dysbiosis triggered by the parasite (Figure 1). These results demonstrated that Giardipain-1-induced alterations seen in the intestinal epithelial cells and in the microbiome contribute to the pathogenesis of giardiasis and its associated complications [30]. However, experimental evidence indicates that it is possible that other major secreted proteases such as GL50803_16160 and GL50803_16779, which are structurally similar to Giardipain-1, may have similar effects during G. duodenalis infection.
This entry is adapted from the peer-reviewed paper 10.3390/ijms241612850
This entry is offline, you can click here to edit this entry!
