Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Respiratory System
Diseases of the lung account for more than 5 million deaths worldwide and are a healthcare burden. Improving clinical outcomes, including mortality and quality of life, involves a holistic understanding of the disease, which can be provided by the integration of lung multi-omics data. An enhanced understanding of comprehensive multiomic datasets provides opportunities to leverage those datasets to inform the treatment and prevention of lung diseases by classifying severity, prognostication, and discovery of biomarkers.
- multiomics
- lung
- lung disease
- lung cancer
- machine learning
- organoid models
- animal models
1. Introduction
Respiratory diseases account for over 5 million deaths yearly, constitute a significant cause of morbidity and are a huge burden to healthcare systems worldwide [1,2]. Annually, about three million deaths are due to chronic obstructive pulmonary disease and asthma (the most common disease) accounts for half a million deaths. Other pulmonary diseases with chronic inflammation and obstruction and often exacerbated by infection include cystic fibrosis, idiopathic pulmonary fibrosis, ciliary dyskinesia, and pneumonia are also leading causes of death and lung cancer leads to the cancer-related deaths category [1,2].
With the ongoing global pandemic due to SARS-COVID-19 [3], respiratory diseases remain a leading cause of death and disability. Recent advances in high-throughput technologies have provided access to multiomics biological data, including genomics, epigenomics, transcriptomics, proteomics, metabolomics and immunomics, and provide a holistic view of pathophysiology in lung disease [4]. Multiomics data give a comprehensive overview of cellular processes (e.g., gene transcription, protein translation or epigenetic processes) associated with a disease and an insight into the complexity of the disease. Biological insights from multi-omics can be integrated with clinical and social data and applied in the clinical setting for improved health outcomes. Single omics are limited by providing associations, whereas multiomic integrations result in deriving a holistic insight that generates testable hypotheses about mechanisms.
State-of-the-art machine-learning methods can integrate high dimensional omics datasets resulting in the ability to predict short- and long-term health trajectories and enable early timely interventions that alter the health course towards better outcomes (precision medicine) [5]. Large datasets such as the omics dataset rely on ‘deep learning’ based on neural networks loosely modeled after neurons of the brain [6]. The insights gained by deep learning of multiomic datasets enhance personalized healthcare decision-making (precision medicine) and biomarker discovery [5,7].
2. Lung Multiomics Models
In almost all respiratory diseases, the epithelium, a monolayer of cells which comprises the lining of the conducting and respiratory airways, is damaged. This compromises the proximal airways’ ability to warm, humidify, and cleanse the inhaled air and distal air to facilitate gas exchange. As a result, health and quality of life are severely impacted by the impaired lung function that occurs in respiratory disease. Human models, such as primary cells and organoids, and animal studies involving integrated multi-omics will allow differences in markers and biological processes between disease and non-disease models to be elucidated (Figure 1). These differences will provide insight into lung disease, including pathways that result in regeneration and repair. Understanding these pathways will be critical in developing preventive treatments and therapeutic modalities to treat lung diseases. It can eventually be harnessed to develop a personalized approach to treating respiratory diseases.
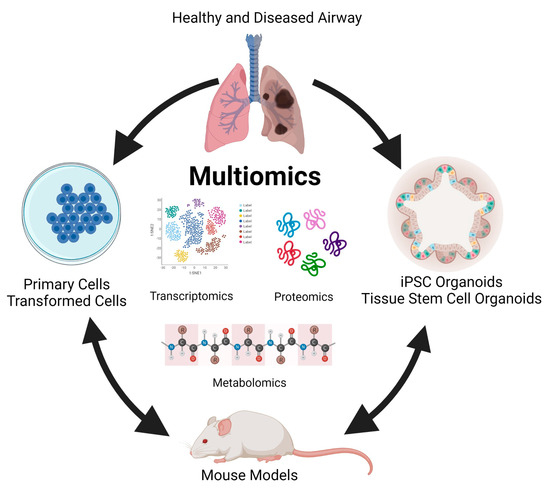
Figure 1. Modelling multi-omics studies in the study of human lung disease.
In recent decades, primary cells and transformed or tumor cell lines have been used to investigate lung diseases. The cells in these models retain many donor tissue characteristics and recapitulate markers and functions present in vivo [49,50,51]. These models have the advantage that they are amenable to genetic engineering, allowing the dissection of the role of individual molecules and pathways in disease [52]. Additionally, the ease of genetic engineering in these systems has allowed testing function via inducible gene expression [53]. Because of their wide use, many of these cell lines are well characterized, providing a foundation for multi-omics studies. Studies in cell lines are well suited for high throughput drug screening and evaluation of drug response [54] and are particularly valuable in studying lung cancer [55,56]. However, these models are not without limitations.
First and foremost, they fall short of replicating the complex nature of many respiratory diseases. Many lack the multiple cell types and cellular polarity present in the proximal and distal respiratory epithelium and exhibit an absence of morphology and structural features that play a significant role in lung biology. These cell lines also lack an immune cell component, which plays an important role in the etiology of many lung diseases [57]. Coupled with questions about the relevance of findings using these models, technical issues, including a requirement for tissue donors, a finite lifespan, and limited expansion capacity, have contributed to a reduced focus on using multi-omics in these models to study many respiratory diseases.
More recently, organoid models have come to the forefront of multi-omic studies of respiratory disorders. Organoids can be derived from either induced pluripotent stem cells or embryonic stem cells (hereafter referred to as iPSC organoids) or established from tissue-derived multipotent stem cells (referred to as ASC organoids) [58]. Differentiation of iPSC organoids occurs in a multistep process that involves a definitive endoderm stage, anterior foregut stage, and then into NKX2-1+ lung epithelial progenitors [59,60]. ASC organoids are established following mechanical and enzymatic isolation of conducting airway or respiratory epithelium stem cells from lavage, small amounts of native tissue, or biopsy specimen [61,62,63,64]. Both iPSC and ASC organoids rely heavily on manipulating exogenously added growth factors to induce differentiation of the mature polarized airway epithelium and the presence of extracellular matrix such as Matrigel®, synthetic matrices, or decellularized tissue scaffoldings. Both organoid models require cultivation on transwells under air-liquid interface conditions (ALI) where the basal side of the epithelium is in contact with media and the apical side is exposed to air to achieve maximum differentiation potential [63,65]. iPSC and ASC organoids can give rise to alveolar organoid models that recapitulate respiratory epithelium, nasal, trachea, or bronchial organoids that recapitulate conducting airway epithelium, and lung organoids that are a mixture [66,67,68]. Like primary and transformed cell models, organoids are amenable to genetic engineering and can be established from donors with genetic disorders that cause lung disease [69]. Organoids are well suited for drug screening and as models for infectious disease research [70,71,72,73] and recapitulate many aspects of other chronic lung diseases such as idiopathic pulmonary fibrosis [74] and cancer [63]. However, iPSC-derived airway cells do not seem to achieve the maturation levels observed in the human lung [75], although ASC organoids seem to contain mature epithelial cells, they lack stromal components such as the immune system that play a significant role in most lung diseases. However, the increased cellular complexity and the modeling of human epithelium combined with a forward-thinking multi-omics approach provide an area for advancement in information surrounding respiratory illness with significant translational potential.
Reports of machine learning integrated multiomics in organoid models and animal models have been published. One organoid model investigated the tumorigenic potential of alveolar type 2 cells (cells of origin of lung adenocarcinoma) using a multiomics and unsupervised machine learning approach [76]. The findings highlight the utility of understanding chromatin regulation in the early oncogenic versions of epithelial cells, which may reveal more effective means to intervene in the progression of Kras-driven lung cancer. In a murine model, authors investigated the interplay of the gut microbiome, metabolism, and host inflammation in obesity-associated asthma using a multiomics approach (microbiome, metabolomics and proteomics) to profile the gut-lung axis in the setting of allergic airway disease and diet-induced obesity [77]. The authors reported that changes to structural proteins in the lung airways and parenchyma may contribute to heightened lung elastance and serve as a potential therapeutic target for obese allergic asthma.
There are many animal models of lung diseases, including chronic diseases such as cystic fibrosis [78], idiopathic pulmonary fibrosis [79,80], viral and bacterial infections [81], and cancer [82]. Animal models of respiratory diseases offer several advantages including reproducibility, control of environmental factors, unlimited numbers of replicates, genetic phenotyping, and accessibility to lung tissue. Multi-omics approaches can be easily used to provide insight into the relationship between environmental stressors and the effect of the stressor on respiratory disease. The information gained can lead to detailed physiologic and pathologic pathways contributing to disease pathogenesis. Animal models of lung disease are instrumental in assessing the predisposition of genetic mutations in causing a specific disease and provide a model in which interactions between components of the whole system can also be examined [83]. Animal models are limited because, in most cases, there are significant differences between human lung tissue and animal lung tissue [44,84]. In addition, many human respiratory diseases are not recapitulated in animal models, and clinical manifestations are difficult to assess. However, comparisons between human and animal multi-omics analyses can validate animal models. Together, multi-omic-based approaches combining data collected from human in vitro and animal in vivo models will provide robustness, rigor, and reproducibility to support drawn conclusions.
This entry is adapted from the peer-reviewed paper 10.3390/microorganisms11082116
This entry is offline, you can click here to edit this entry!
