Dietary components are essential for the structural and functional development of the brain. Among these, docosahexaenoic acid, 22:6n-3 (DHA), is critically necessary for the structure and development of the growing fetal brain in utero.
- Docosahexaenoic Acid
- DHA
- long-chain polyunsaturated fatty acid
- brain
- fetus
- infant
- placenta
- breast milk
1. Introduction
Docosahexaenoic acid, 22:6n-3(DHA), an n-3 (omega-3) long-chain polyunsaturated fatty acid (LCPUFA), plays various essential roles in human health [1][2][3][4]. DHA takes part in several biological processes, such as angiogenesis, immune modulation, inflammatory response, signal transduction, apoptosis, cell proliferation, and a host of the membrane, cellular and molecular functions that affect health and diseases [3][5][6]. The DHA metabolites also mediate their roles in cellular signaling processes [7]. Inadequate dietary consumption of DHA and eicosapentaenoic acid, 22:5n-3 (EPA) impairs the optimal growth of the feto-placental unit and imposes plausible risks of cognitive decline, inflammatory disease, cardiovascular disease, inflammatory disorders, behavioral changes, and mental stress in later life [5][8][9]. While the metabolites of EPA and DHA, such as eicosanoids and docosanoids, are involved in cell signaling, DHA is primarily used for membrane structure and function [3][4]. Modern diets are mostly deficient in n-3 PUFAs, leading to sub-optimal organ function that may predispose individuals to an increased risk of diseases [9][10][11].
The recommended intake of DHA and the EPA is based on the quantum of data supporting beneficial outcomes in protecting cognitive development and cardiovascular diseases. Experts are agreed upon to enhance n-3 LCPUFA intake via increased seafood or supplementary approaches. An n-3 LCPUFA intake of no less than 250–500 mg/d should be made available to maintain healthy adults’ physiological needs in their daily routine. Even though DHA consumption during pregnancy is an essential consideration as it is required for fetal brain development and growth, a limited number of countries have adopted and implemented appropriate guidelines for the n-3 LCPUFA consumption during pregnancy. DHA’s requirement during placental growth and early development has been highlighted recently. Thus, low maternal DHA status may lead to the placenta’s functional inadequacy and accordingly alter fetoplacental growth and development. Therefore, DHA supplementation well before gestation can be considered to prevent feto-placental associated developmental disorders.
2. The DHA Uptake System in the Human Brain
Usually, non-esterified DHA is the major plasma pool for its supply to the brain [12][13]. DHA is also present as an esterified to lysophosphatidylcholine (LPC-DHA) pools in similar amounts. The brain maintains its fatty acid level via fatty acid uptake from circulation [14]. Astrocytes and endothelial cells of the blood-brain barrier (BBB) are significantly involved in the brain’s uptake of plasma fatty acids. The brain’s fatty acid uptake may occur via different mechanisms, such as passive diffusion and a saturable protein-mediated transport system.
In circulation, free fatty acids (FFAs) are mainly bound by albumin. At the inner surface of endothelial cell membranes, a small fraction of FFAs is delivered into the subcellular compartments for further metabolism. At the same time, most FFAs diffuse into the cytosol with or without the help of the battery of cell membrane- and cytoplasmic fatty acid-binding proteins. Figure 1 shows the putative fatty acid transport system of the brain. There are four classes of fatty acid transport proteins involved in transportation in the adult brain, including fatty acid translocase (FAT/CD36), plasma membrane-fatty acid-binding proteins (FABPpm), fatty acid transport proteins (FATPs), and cytoplasmic FABPs. Additionally, MFSD2a is newly identified as a DHA transporter in the brain. Even though there is a preferential uptake by the brain of esterified DHA (LPC-DHA), this is not the major pathway by which DHA is transported into the brain. Despite being an abundant fatty acid in brain phospholipids, DHA cannot be synthesized de novo in the brain and must be imported across the blood-brain barrier, but its transportation pathways are unknown yet. MFSD2a is the major DHA transporter, is expressed exclusively in the endothelium of the BBB of micro-vessels. The MFSD2a-deficient mice brain had significantly reduced DHA concentrations with neuronal cell loss in the hippocampus and cerebellum. These mice also exhibited had microcephaly with severe cognitive deficits and anxiety. MFSD2a transports LPC-DHA, but not unesterified DHA, in a sodium-dependent manner. Notably, MFSD2a transports plasma pool LPCs carrying long-chain fatty acids (C>14). Long-chain acyl-CoA synthetases (ACSLs) are also involved in brain DHA uptake [15][16]. Brain development requires an incredible increase in the de novo synthesis and accretion of DHA, mediated by several factors, including sterol regulatory element-binding protein SREBP. In normal physiology, the activity of MFSD2a is regulated by SREBP to maintain a balance between de novo lipogenesis and exogenous uptake of LPC-DHA [17].
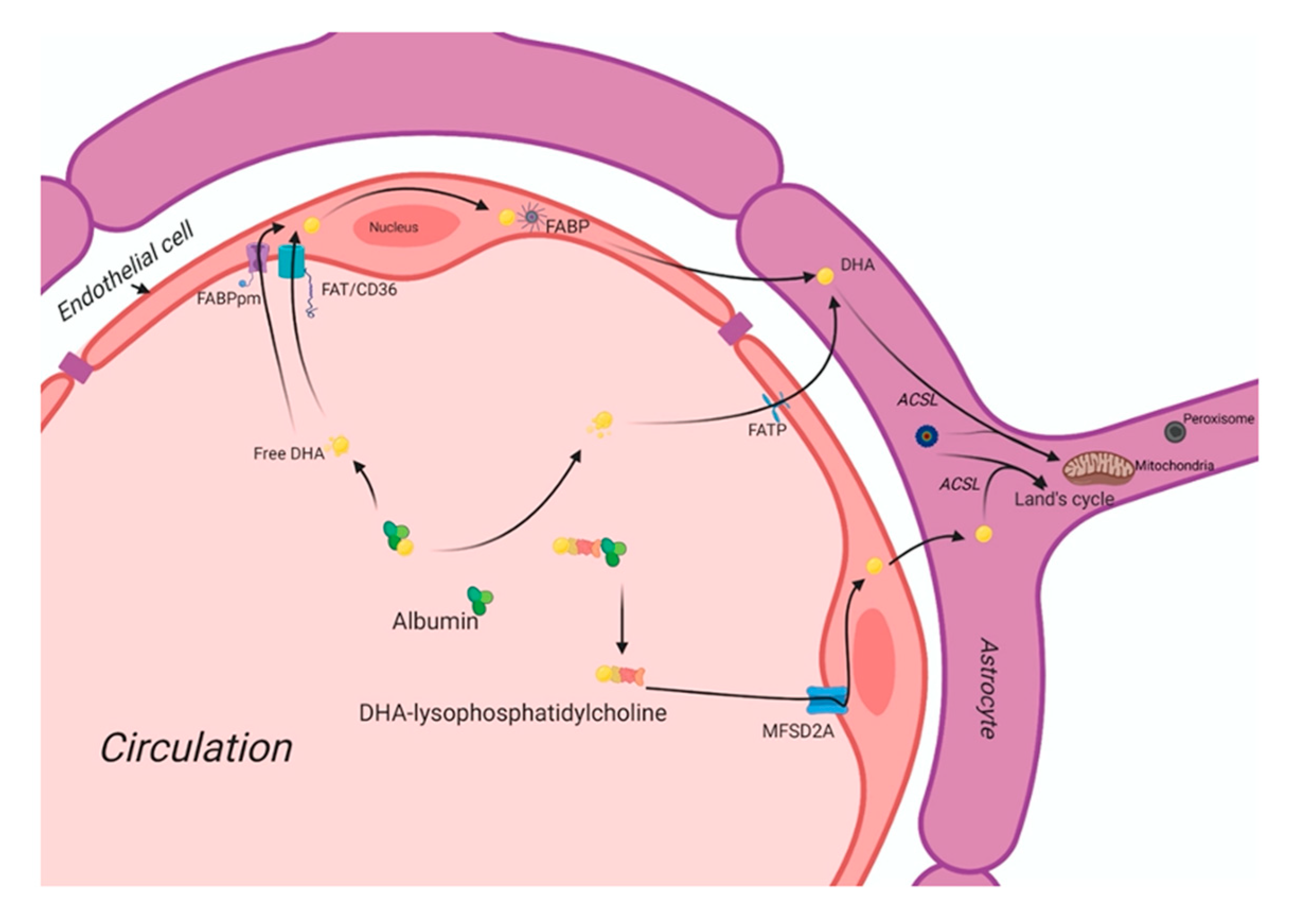
DHA is carried through the plasma by albumin and circulating lipoproteins. There are four classes of lipid transport proteins involved in DHA uptake of the brain that includes fatty acid translocase (FAT/CD36), plasma membrane fatty acid-transport proteins (FABPpm), and fatty acid transport proteins (FATPs) and cytoplasmic FABPs. MFSD2a, a specific protein, can transport plasma LPC-DHA, but not other DHA forms, across the blood-brain barrier to the neuron.
DHA Deficiency during Fetal Brain Development and Its Impact on Cognitive Functions
During pregnancy and lactation, maternal intake of DHA ensures adequate maternal reserve deposited during pregnancy to support six-month breast-fed post-natal life. Maternal adipose resources during pregnancy cover the increasing demand for DHA in the early post-natal stage. Although it is difficult to estimate the quantity of DHA require in the diet for optimum brain development, the study from Kuiper et al. [18] first estimated the absolute requirement of DHA, ARA, and LA at 25 (conceptual age), 35 (preterm), and 45 (term) weeks. Based on the mother’s data fed on the western diet, the DHA accretion rate was found 42 mg/day in the last five weeks of pregnancy. The accretion of DHA was found double in the last five weeks of pregnancy compared with the first thirty-five weeks together. The DHA accretion rate largely determines the DHA deficiency during brain development in the brain during term and preterm conditions. Thus, it is important to devise the DHA requirement for preterm babies based on the mother’s data from a different ethnic and dietary background. The majority of the available data suggests DHA’s optimal requirement during brain development in infants is fed on the Western diet. A scenario with high LA intake in the diet will further enhance the need for pre-formed DHA in infant formula. To fulfill enough maternal reserve of DHA, intake may be required well before conception.
In developing populations, where pregnant women’s usual diet is low in fats in which n-6 PUFAs are predominantly present with little intake of ALA or DHA as n-3PUFAs, most of the women start pregnancy with inadequate or insufficient n-3 PUFA status in their reserve. Under such a scenario, where a maternal reserve of DHA is low, endogenous synthesis of DHA from precursors is inadequate, resulting in an insufficient supply of DHA to the fetus that may affect DHA accretion in the brain during the brain development phase of the neonate. DHA’s placental deficiency can be correlated with the lower DHA accretion in the brain of the babies born preterm in the developing population. There is no clinical data available about DHA accretion in the brain and neonatal’s cognitive performance from the mother fed on the n-3 deficient diet in such a population. Therefore, optimal maternal intake of n-3 LCPUFAs is essential to fulfilling DHA’s neonatal requirement for the first six months. Maternal intake of DHA is correlated with the problem-solving skills of the children in some aspects. It was argued that inadequate intake of DHA could affect rapid accretion of DHA in the human brain, mainly when brain growth is maximal as with the third trimester or first six months of life. Adequate DHA during this period ensures the maturation of the prefrontal cortex’s specific brain domain that may support the problem-solving skills.
The impact of DHA deficiencies on cognitive functions is extensively studied using animal models in vivo. DHA deficiency can influence DHA’s endogenous synthesis in the brain and its impact on synaptic plasticity. A recent study with DHA deficient mice by silencing ELOVL2, an enzyme that is responsible for endogenous synthesis of DHA from its precursors, showed downregulation in the expression of neural plasticity factors (BDNF, Arc-1) with a concomitant increase in the pro-inflammatory markers (TNFα, IL-1β, inducible nitric oxide synthase iNOS) in the cerebral cortex of the DHA deficient mice [19]. The altered expression of these factors is also associated with the learning and memory function in the brain. The endogenous DHA deficient mice showed an alteration in microglial architecture and cytokine factors without involving astrocytes indicates that resident immune cells are affected in the brain by endogenous DHA deficiency. The replenishment with DHA restored the physiological expression of neuroinflammatory and neuroplasticity factors in the cerebral cortex. These data indicate that DHA plays a critical role in neuroimmune communication in brain function and synaptic plasticity.
The damaging effects of n-3 PUFA deficiency on brain lipid composition and memory performance were evidenced in lipopolysaccharides (LPS)-induced rat models, suggesting that in utero n-3 PUFAs deficiency could be a potential risk factor for neurodevelopmental disorders [20]. The n-3 PUFA deficiency in rats shows downregulated glutamate receptors and upregulated pro-inflammatory TNFα gene expression in the central nervous tissue independent of their effects on membrane composition [21]. Chronic deficiency of DHA over multiple generations during brain spurt affects the process of neurodevelopment by modulating the neuronal cell growth and differentiation, as well as neuronal signaling. The deficiency may cause a functional deficit in the offspring’s learning and cognitive efficiency by reducing intellectual potential and enhancing the risks of neurological diseases in adult life [22]. The n-3 fatty acid deficiency disrupts the peripheral balance of pro-and anti-inflammatory states in the brain due to altered systemic ARA: DHA ratio [23]. The excess ARA generates high prostaglandin concentrations, leukotriene, and thromboxane that lead to a pro-inflammatory state in the brain that disrupts the balance of anti- (n-3) and pro-inflammatory (n-6) eicosanoids due to alteration in GPR receptors’ signaling [24]. In the animal model, DHA’s maternal deficiency revealed reduced telencephalon structure in the hippocampus region [25]. Such a deficiency state affects region-specific brain development areas where the cerebral frontal cortex region is affected mostly, leading to hyper motor activity, reduced learning ability, and altered monoamine transmission [26]. The maternal DHA deficiency affects the offspring’s brain development in a gender-specific manner due to differential efficiency of endogenous DHA converting enzyme in males and females.
As the endogenous conversion of DHA from its precursor is more efficient in female newborns due to estrogen presence, the infant male is more susceptible to the risk of brain disorders, such as ADHD, Autism, etc., in their later life [27]. The maternal DHA deficiency state profoundly affects behavioral change in feed intake, anxiety, and stress response in the offspring [28]. The n-3 deficiency state triggers sucrose-motivated food intake preference due to alteration in the brain-rewarding pathway that may prompt children to consume calorie-dense foods [29]. Chronic deficiency of n-3 fatty acids for multiple generations induces anxiety-related stress behavior in the offspring due to altered expression of neuropeptide Y-1 receptor and glucocorticoid receptor in the pre-frontal and hippocampus of the rat brain [22][30]. Data from several in vivo studies suggest that DHA promotes neurogenesis by improving the membrane fluidity in the structural domain of the hippocampus, prefrontal cortex, and hypothalamus region to stabilize the neurodevelopment circuitry network required for learning and memory recognition processes [31]. N-3 fatty acid deficiency during utero development and in the postnatal state negatively impacts cognitive abilities [32][33]. DHA’s dietary deficiency increases the risk for neurocognitive disorders, whereas a diet enriched with DHA increases learning and memory and protects against cognitive decline during aging. However, whether increased intake of DHA can prevent the risk of brain disorders requires further investigation.
This entry is adapted from the peer-reviewed paper 10.3390/nu12123615
References
- Salem, N., Jr.; Litman, B.; Kim, H.Y.; Gawrisch, K. Mechanisms of action of docosahexaenoic acid in the nervous system. Lipids 2001, 36, 945–959.
- Bourre, J.M. Dietary omega-3 fatty acids for women. Biomed. Pharm. 2007, 61, 105–112.
- Bradbury, J. Docosahexaenoic acid (DHA): An ancient nutrient for the modern human brain. Nutrients 2011, 3, 529–554.
- Horrocks, L.A.; Yeo, Y.K. Health benefits of docosahexaenoic acid (DHA). Pharmacol. Res. 1999, 40, 211–225.
- Quinn, J.F.; Raman, R.; Thomas, R.G.; Yurko-Mauro, K.; Nelson, E.B.; Van Dyck, C.; Galvin, J.E.; Emond, J.; Jack, C.R., Jr.; Weiner, M.; et al. Docosahexaenoic acid supplementation and cognitive decline in Alzheimer disease: A randomized trial. J. Am. Med. Assoc. 2010, 304, 1903–1911.
- de Urquiza, A.M.; Liu, S.; Sjoberg, M.; Zetterstrom, R.H.; Griffiths, W.; Sjovall, J.; Perlmann, T. Docosahexaenoic acid, a ligand for the retinoid X receptor in mouse brain. Science 2000, 290, 2140–2144.
- Ji, R.R.; Xu, Z.Z.; Strichartz, G.; Serhan, C.N. Emerging roles of resolvins in the resolution of inflammation and pain. Trends Neurosci. 2011, 34, 599–609.
- Matesanz, N.; Park, G.; McAllister, H.; Leahey, W.; Devine, A.; McVeigh, G.E.; Gardiner, T.A.; McDonald, D.M. Docosahexaenoic acid improves the nitroso-redox balance and reduces VEGF-mediated angiogenic signaling in microvascular endothelial cells. Invest. Ophthalmol. Vis. Sci. 2010, 51, 6815–6825.
- Kabaran, S.; Besler, H.T. Do fatty acids affect fetal programming? J. Health Popul. Nutr. 2015, 33, 14.
- Basak, S.; Vilasagaram, S.; Duttaroy, A.K. Maternal dietary deficiency of n-3 fatty acids affects metabolic and epigenetic phenotypes of the developing fetus. Prostaglandins Leukot. Essent. Fatty Acids 2020, 158, 102109.
- Lacombe, R.J.S.; Chouinard-Watkins, R.; Bazinet, R.P. Brain docosahexaenoic acid uptake and metabolism. Mol. Asp. Med. 2018, 64, 109–134.
- Lagarde, M.; Bernoud, N.; Brossard, N.; Lemaitre-Delaunay, D.; Thies, F.; Croset, M.; Lecerf, J. Lysophosphatidylcholine as a preferred carrier form of docosahexaenoic acid to the brain. J. Mol. Neurosci. 2001, 16, 201–204.
- Chen, C.T.; Kitson, A.P.; Hopperton, K.E.; Domenichiello, A.F.; Trepanier, M.O.; Lin, L.E.; Ermini, L.; Post, M.; Thies, F.; Bazinet, R.P. Plasma non-esterified docosahexaenoic acid is the major pool supplying the brain. Sci. Rep. 2015, 5, 15791.
- Demar, J.C., Jr.; Ma, K.; Chang, L.; Bell, J.M.; Rapoport, S.I. alpha-Linolenic acid does not contribute appreciably to docosahexaenoic acid within brain phospholipids of adult rats fed a diet enriched in docosahexaenoic acid. J. Neurochem. 2005, 94, 1063–1076.
- Duttaroy, A.K. Transport of fatty acids across the human placenta: A review. Prog. Lipid Res. 2009, 48, 52–61.
- Campbell, F.M.; Dutta-Roy, A.K. Plasma membrane fatty acid-binding protein (FABPpm) is exclusively located in the maternal facing membranes of the human placenta. FEBS Lett. 1995, 375, 227–230.
- Chan, J.P.; Wong, B.H.; Chin, C.F.; Galam, D.L.A.; Foo, J.C.; Wong, L.C.; Ghosh, S.; Wenk, M.R.; Cazenave-Gassiot, A.; Silver, D.L. The lysolipid transporter Mfsd2a regulates lipogenesis in the developing brain. PLoS Biol. 2018, 16, e2006443.
- Kuipers, R.S.; Luxwolda, M.F.; Offringa, P.J.; Boersma, E.R.; Dijck-Brouwer, D.A.; Muskiet, F.A. Fetal intrauterine whole body linoleic, arachidonic and docosahexaenoic acid contents and accretion rates. Prostaglandins Leukot. Essent. Fat. Acids 2012, 86, 13–20.
- Talamonti, E.; Sasso, V.; To, H.; Haslam, R.P.; Napier, J.A.; Ulfhake, B.; Pernold, K.; Asadi, A.; Hessa, T.; Jacobsson, A.; et al. Impairment of DHA synthesis alters the expression of neuronal plasticity markers and the brain inflammatory status in mice. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2020, 34, 2024–2040.
- Labrousse, V.F.; Leyrolle, Q.; Amadieu, C.; Aubert, A.; Sere, A.; Coutureau, E.; Grégoire, S.; Bretillon, L.; Pallet, V.; Gressens, P.; et al. Dietary omega-3 deficiency exacerbates inflammation and reveals spatial memory deficits in mice exposed to lipopolysaccharide during gestation. Brain Behav. Immun. 2018, 73, 427–440.
- Kitajka, K.; Sinclair, A.J.; Weisinger, R.S.; Weisinger, H.S.; Mathai, M.; Jayasooriya, A.P.; Halver, J.E.; Puskás, L.G. Effects of dietary omega-3 polyunsaturated fatty acids on brain gene expression. Proc. Natl. Acad. Sci. USA 2004, 101, 10931–10936.
- Bhatia, H.S.; Agrawal, R.; Sharma, S.; Huo, Y.X.; Ying, Z.; Gomez-Pinilla, F. Omega-3 fatty acid deficiency during brain maturation reduces neuronal and behavioral plasticity in adulthood. PLoS ONE 2011, 6, e28451.
- Tian, C.; Fan, C.; Liu, X.; Xu, F.; Qi, K. Brain histological changes in young mice submitted to diets with different ratios of n-6/n-3 polyunsaturated fatty acids during maternal pregnancy and lactation. Clin. Nutr. 2011, 30, 659–667.
- Dragano, N.R.V.; Solon, C.; Ramalho, A.F.; de Moura, R.F.; Razolli, D.S.; Christiansen, E.; Azevedo, C.; Ulven, T.; Velloso, L.A. Polyunsaturated fatty acid receptors, GPR40 and GPR120, are expressed in the hypothalamus and control energy homeostasis and inflammation. J. Neuroinflamm. 2017, 14, 91.
- Coti Bertrand, P.; O’Kusky, J.R.; Innis, S.M. Maternal dietary (n-3) fatty acid deficiency alters neurogenesis in the embryonic rat brain. J. Nutr. 2006, 136, 1570–1575.
- Carrie, I.; Clement, M.; de Javel, D.; Frances, H.; Bourre, J.M. Specific phospholipid fatty acid composition of brain regions in mice. Effects of n-3 polyunsaturated fatty acid deficiency and phospholipid supplementation. J. Lipid Res. 2000, 41, 465–472.
- Giltay, E.J.; Gooren, L.J.G.; Toorians, A.W.F.T.; Katan, M.B.; Zock, P.L. Docosahexaenoic acid concentrations are higher in women than in men because of estrogenic effects. Am. J. Clin. Nutr. 2004, 80, 1167–1174.
- Bondi, C.O.; Taha, A.Y.; Tock, J.L.; Totah, N.K.; Cheon, Y.; Torres, G.E.; Rapoport, S.I.; Moghaddam, B. Adolescent behavior and dopamine availability are uniquely sensitive to dietary omega-3 fatty acid deficiency. Biol. Psychiatry 2014, 75, 38–46.
- Auguste, S.; Sharma, S.; Fisette, A.; Fernandes, M.F.; Daneault, C.; Des Rosiers, C.; Fulton, S. Perinatal deficiency in dietary omega-3 fatty acids potentiates sucrose reward and diet-induced obesity in mice. Int. J. Dev. Neurosci. Off. J. Int. Soc. Dev. Neurosci. 2018, 64, 8–13.
- Harauma, A.; Moriguchi, T. Dietary n-3 fatty acid deficiency in mice enhances anxiety induced by chronic mild stress. Lipids 2011, 46, 409–416.
- Kawakita, E.; Hashimoto, M.; Shido, O. Docosahexaenoic acid promotes neurogenesis in vitro and in vivo. Neuroscience 2006, 139, 991–997.
- Henriksen, C.; Haugholt, K.; Lindgren, M.; Aurvag, A.K.; Ronnestad, A.; Gronn, M.; Solberg, R.; Moen, A.; Nakstad, B.; Berge, R.K.; et al. Improved cognitive development among preterm infants attributable to early supplementation of human milk with docosahexaenoic acid and arachidonic acid. Pediatrics 2008, 121, 1137–1145.
- Moriguchi, T.; Salem, N., Jr. Recovery of brain docosahexaenoate leads to recovery of spatial task performance. J. Neurochem. 2003, 87, 297–309.
