Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Volumetric absorptive microsampling (VAMS) is the newest and most promising sample-collection technique for quantitatively analyzing drugs, especially for routine therapeutic drug monitoring (TDM) and pharmacokinetic studies. This technique uses an absorbent white tip to absorb a fixed volume of a sample (10–50 µL) within a few seconds (2–4 s), is more flexible, practical, and more straightforward to be applied in the field, and is probably more cost-effective than conventional venous sampling (CVS).
- volumetric absorptive microsampling (VAMS)
- conventional venous sampling (CVS)
- therapeutic drug monitoring
- pharmacokinetic studies
- drug concentration
1. Introduction
The essential goal of clinical pharmacology is to understand the dose–concentration–effect relationship. The study of pharmacokinetics seeks to explain the time course of drug concentrations in the body (dose–concentration relationship). However, the time course of drug concentrations cannot predict the magnitude of the drug effect (concentration–effect relationship) [1][2]. This dose–concentration–effect relationship is now used as the central concept of therapeutic drug monitoring (TDM), which individualizes drug dosage by attaining specific target plasma concentrations (therapeutic ranges) guided by the measurement of plasma drug concentrations [3]. TDM is used for assessing efficacy, diagnosing undertreatment, preventing adverse effects, guidance to stop the treatment, monitoring and detecting drug interaction, and monitoring adherence [4]. It is recommended for drugs with a narrow therapeutic range, significant pharmacokinetic variability, and a clear relationship between drug concentrations and clinical response [5].
The sampling technique commonly used to routinely measure drug concentration, either for TDM or pharmacokinetic studies, is conventional venous sampling (CVS), which draws blood from the vein. CVS can lead to unexpected complications, such as hematoma and thrombophlebitis [6][7]. This technique requires much blood for each sample (up to 5 mL), is invasive and inconvenient, has a relatively high cost, sometimes needs special conditions (a cold chain) for transport, and samples must be centrifuged before storage as plasma or serum. If the sample is not immediately analyzed, it must be frozen at −20 up to −80 °C. These characteristics cause CVS to be less flexible for application in the field [8][9][10]. An advantage of CVS is that the sample volume is large, while analysis only needs a small volume; therefore, the leftover sample could be stored and re-analyzed [6][7].
Considering the many limitations of CVS, which can complicate TDM programs or pharmacokinetic studies, there have been many innovations in sampling techniques for drug concentration measurement in the past decade, called microsampling techniques. Microsampling is a sampling technique that only takes a small volume of samples (<100 µL for blood) from the body for analysis; it is less invasive, less painful, and more efficient. This technique is also more practical compared to CVS [11]. Therefore, this technique may improve routine clinical care in TDM and benefit pharmacokinetic studies, including those with children as subjects. Some microsampling techniques that have already been developed are capillary microsampling (CMS), dried blood spots (DBS), dried plasma spots (DPS), plasma preparation technologies, solid-phase microextraction (SPME), and volumetric absorptive microsampling (VAMS) [12]. VAMS and DBS have been the most researched to analyze drugs [12][13][14][15][16][17]. Previously, the most commonly used and straightforward microsampling design was DBS, which requires small volume sizes (30 µL per spot). But DBS has some limitations primarily related to the effect of hematocrit values that may affect measured concentrations. The VAMS technique was then developed to tide over the boundaries of DBS [12][18][19]. VAMS is a promising sample-collection technique for the quantitative analysis of drugs, which only takes a 10–50 µL volume of samples by an absorbent tip [20][21]. Compared to CVS, the VAMS technique has some superiority in practicality and flexibility of the sampling process because it only needs a small sample volume. The sampling process is relatively fast and less invasive and there is no need to freeze the sample before transport; thus, it may be more cost-effective, there is no need for centrifugation before storage, and it also reduces the amount of freezers needed to keep samples in the long term [18][19][22]. Therefore, this VAMS technique is more flexible for application in the field than CVS and can be innovative for sampling processes that are more practical and simpler [18][22].
2. Sampling Procedure with VAMS
VAMS is the newest innovation in microsampling techniques. VAMS was designed to absorb a minimal and fixed volume, such as 10, 20, and 50 µL. VAMS devices contain a white hydrophilic pore absorbent tip attached to a plastic handler [19]. The picture of the VAMS device is shown in Figure 1, which comes with two different types of devices, namely clamshells and cartridges. The sampling procedure with VAMS is simple: dipping the tip of VAMS in the location that has been punctured using a lancet at a 45° angle for 2–4 s until the tip is entirely red. The sample for this device is not only blood; VAMS has also been used to collect urine, saliva, or other liquid biological samples [18][19]. After obtaining the sample, the devices are dried at room temperature for at least 2 h. The samples can be transferred at room temperature for storage or analysis [19][23]. The drying of the devices could correlate with the limitation of VAMS itself. Care is also taken to ensure that the tips do not touch either other tips or their surroundings to prevent blood transfer during drying. If it touches others, it could be contaminated. Also, the variation in drying times could be an issue with reproducible recovery. Drug extraction is more inconvenient when samples become drier. Understanding the effect of drying on VAMS samples is essential for method development and validation. The total time for drying depends on the size of the absorbent tip on the VAMS devices. It has been known that 1 h of drying at room temperature is adequate when using 10 µL VAMS. Furthermore, the drying time also could generate the degradation of the sample; thus, the stability of analytics in drying time needs to be evaluated [18][24].
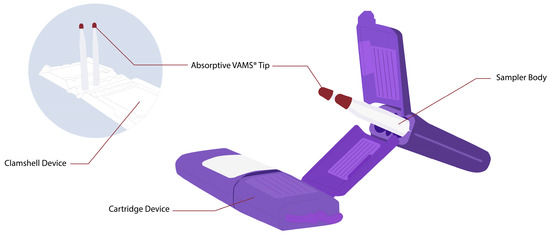
Figure 1. Volumetric absorptive microsampling devices (left picture is VAMS in clamshell device; right picture is VAMS in cartridge device).
The dried sample from the VAMS technique has better stability than a liquid sample. Some studies reported that the desired analyte was stable for over a month in dried conditions at room temperature for an extended period (more than one month) [25][26][27][28][29][30][31][32][33][34]. Storage conditions need to be considered for the samples obtained from the VAMS technique, such as storing them in a closed and dark container and having a desiccant to prevent degradation [22]. The VAMS manufacturer provides clamshell storage with a hole for airflow that is important for drying. If the appropriate storage conditions are applied, it could extend the stability of the drugs [16][22][35]. However, stability still depends on many factors, such as analyte and storage conditions (temperature and how to save the sample). In the VAMS device, samples need to be stored in a tightly closed container that is dark and possibly with desiccant. Therefore, stability parameters must be evaluated during analytical method validation [22].
For the sample analysis, sample preparation must be conducted for the extraction process. Before the extraction, the tip from VAMS devices should be released from its handler [13][19][22][23][25], or the whole VAMS device could be used using automatic machines [19][22][36] to overcome the hematocrit issue of DBS [19]. The choice of type and solvent volume, extraction time, and extraction procedure are the most important in sample preparation for the VAMS technique. Ideally, a suitable extraction procedure is a procedure that can result in high reproducibility, maximal recovery of drugs, and minimal matrix effect [19][37]. In studies that used the VAMS technique, the type of solvent varied from 100% water [27][35][38][39][40] to 100% organic solvent [13][23][28][29][36][41][42][43][44][45]. Acid or base could be added to increase recovery [13][25][37][39][40][43][46], and the extraction time varied from minutes to one hour. A longer extraction time could increase the analyte’s recovery [19][36]. The VAMS technique, since it is a microsampling device, has a sensitivity issue based on its volume. It is known that the reduced sample volume impacts the sensitivity since the smaller volume also leads to small analyte/concentrations. Nevertheless, the small blood volume is sufficient for clinical assays because modern instruments are highly sensitive and can detect low concentrations of drugs and metabolites. Consequently, this highly sensitive instrument could overcome the issue of sensitivity of small-volume samples, especially in VAMS [24][47].
Assays for many drugs taken by VAMS have been optimized and analytically validated, such as cefepime, midazolam, tacrolimus, paracetamol, and voriconazole. Yet, only a few assays underwent clinical validations [23][48][49][50][51]. Some studies comparing VAMS and CVS demonstrated a strong correlation of drug concentrations from samples obtained by both sampling techniques, for example, lamotrigine, levetiracetam, phenytoin, valproic acid, and albendazole (r > 0.95) [48][49].
This entry is adapted from the peer-reviewed paper 10.3390/molecules28166046
References
- Aronson, J.K. Concentration-effect and dose-response relations in clinical pharmacology. Br. J. Clin. Pharmacol. 2007, 63, 255–257.
- Holford, N.H.G.; Sheiner, L.B. Understanding the Dose-Effect Relationship. Clin. Pharmacokinet. 1981, 6, 429–453.
- Aarnoutse, R.E.; Schapiro, J.M.; Boucher, C.A.B.; Hekster, Y.A.; Burger, D.M. Therapeutic drug monitoring: An aid to optimising response to antiretroviral drugs? Drugs 2003, 63, 741–753.
- Saleem, M.A.; Basharat, R.; Rana, N.A.; Akhtar Khan Khattak, S. Role of clinician in therapeutic drug monitoring practice. Clin. Pract. 2020, 17, 1429–1435.
- Ghiculesco, R. Abnormal laboratory results: Therapeutic drug monitoring: Which drugs, why, when and how to do it. Aust. Prescr. 2008, 31, 42–44.
- Roberge, R.J. Venodilatation techniques to enhance venepuncture and intravenous cannulation. J. Emerg. Med. 2004, 27, 69–73.
- Buowari, O.Y. Complications of venepuncture. Adv. Biosci. Biotechnol. 2013, 4, 126–128.
- O’Connor, R.; O’Sullivan, J.F.; O’Kennedy, R. Determination of serum and tissue levels of phenazines including clofazimine. J. Chromatogr. B Biomed. Appl. 1996, 681, 307–315.
- Schaad-Lanyi, Z.; Dieterle, W.; Dubois, J.P.; Theobald, W.; Vischer, W. Pharmacokinetics of clofazimine in healthy volunteers. Int. J. Lepr. 1987, 55, 9–15.
- Katzung, B.G. Basic & Clinical Pharmacology, 14th ed.; McGraw Hill Medical: New York, NY, USA, 2018; ISBN 9781259641152.
- Lei, B.U.W.; Prow, T.W. A review of microsampling techniques and their social impact. Biomed. Microdevices 2019, 21, 81.
- Parker, S.L.; Dorofaeff, T.; Lipman, J.; Ballot, D.E.; Bandini, R.M.; Wallis, S.C.; Roberts, J.A. Is there a role for microsampling in antibiotic pharmacokinetic studies? Expert Opin. Drug Metab. Toxicol. 2016, 12, 601–614.
- Ye, Z.; Gao, H. Evaluation of sample extraction methods for minimizing hematocrit effect on whole blood analysis with volumetric absorptive microsampling. Bioanalysis 2017, 9, 349–357.
- Barco, S.; Castagnola, E.; Moscatelli, A.; Rudge, J.; Tripodi, G.; Cangemi, G. Volumetric adsorptive microsampling-liquid chromatography tandem mass spectrometry assay for the simultaneous quantification of four antibiotics in human blood: Method development, validation and comparison with dried blood spot. J. Pharm. Biomed. Anal. 2017, 145, 704–710.
- Koponen, J.; Rudge, J.; Kushon, S.; Kiviranta, H. Novel volumetric adsorptive microsampling technique for determination of perfluorinated compounds in blood. Anal. Biochem. 2018, 545, 49–53.
- D’Urso, A.; Rudge, J.; Patsalos, P.N.; De Grazia, U. Volumetric Absorptive Microsampling: A New Sampling Tool for Therapeutic Drug Monitoring of Antiepileptic Drugs. Ther. Drug Monit. 2019, 41, 681–692.
- Lee, K.; Jun, S.H.; Choi, M.S.; Song, S.H.; Park, J.S.; Lee, J.H.; Park, K.U.; Song, J. Application of the isoniazid assay in dried blood spots using the ultra-performance liquid chromatography-tandem mass spectrometry. Clin. Biochem. 2017, 50, 882–885.
- Denniff, P.; Spooner, N. Volumetric absorptive microsampling: A dried sample collection technique for quantitative bioanalysis. Anal. Chem. 2014, 86, 8489–8495.
- Kok, M.G.M.M.; Fillet, M. Volumetric absorptive microsampling: Current advances and applications. J. Pharm. Biomed. Anal. 2018, 147, 288–296.
- Marchand, A.; Roulland, I.; Semence, F.; Audran, M. Volumetric Absorptive Microsampling (VAMS) technology for IGF-1 quantification by automated chemiluminescent immunoassay in dried blood. Growth Horm. IGF Res. 2019, 50, 27–34.
- Spooner, N.; Denniff, P.; Michielsen, L.; De Vries, R.; Ji, Q.C.; Arnold, M.E.; Woods, K.; Woolf, E.J.; Xu, Y.; Boutet, V.; et al. A device for dried blood microsampling in quantitative bioanalysis: Overcoming the issues associated blood hematocrit. Bioanalysis 2015, 7, 653–659.
- Protti, M.; Mandrioli, R.; Mercolini, L. Tutorial: Volumetric absorptive microsampling (VAMS). Anal. Chim. Acta 2019, 1046, 32–47.
- Denniff, P.; Parry, S.; Dopson, W.; Spooner, N. Quantitative bioanalysis of paracetamol in rats using volumetric absorptive microsampling (VAMS). J. Pharm. Biomed. Anal. 2015, 108, 61–69.
- Moorthy, G.S.; Vedar, C.; Downes, K.J.; Fitzgerald, J.C.; Scheetz, M.H.; Zuppa, A.F. Microsampling Assays for Pharmacokinetic Analysis and Therapeutic Drug Monitoring of Antimicrobial Drugs in Children: A Critical Review. Ther. Drug Monit. 2021, 43, 335–345.
- De Kesel, P.M.M.; Lambert, W.E.; Stove, C.P. Does volumetric absorptive microsampling eliminate the hematocrit bias for caffeine and paraxanthine in dried blood samples? A comparative study. Anal. Chim. Acta 2015, 881, 65–73.
- Marahatta, A.; Megaraj, V.; McGann, P.T.; Ware, R.E.; Setchell, K.D.R. Stable-isotope dilution HPLC-electrospray ionization tandem mass spectrometry method for quantifying hydroxyurea in dried blood samples. Clin. Chem. 2016, 62, 1593–1601.
- Anoshkina, Y.; Costas-Rodríguez, M.; Vanhaecke, F. Iron isotopic analysis of finger-prick and venous blood by multi-collector inductively coupled plasma-mass spectrometry after volumetric absorptive microsampling. J. Anal. At. Spectrom. 2017, 32, 314–321.
- Kip, A.E.; Kiers, K.C.; Rosing, H.; Schellens, J.H.M.; Beijnen, J.H.; Dorlo, T.P.C. Volumetric absorptive microsampling (VAMS) as an alternative to conventional dried blood spots in the quantification of miltefosine in dried blood samples. J. Pharm. Biomed. Anal. 2017, 135, 160–166.
- Protti, M.; Rudge, J.; Sberna, A.E.; Gerra, G.; Mercolini, L. Dried haematic microsamples and LC–MS/MS for the analysis of natural and synthetic cannabinoids. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2017, 1044–1045, 77–86.
- Nys, G.; Cobraiville, G.; Kok, M.G.M.; Wéra, O.; Servais, A.C.; Fillet, M. Comparison of nanofluidic and ultra-high performance liquid chromatography-tandem mass spectrometry for high sensitive pharmacokinetic studies of estrogens starting from whole blood microsampling. J. Chromatogr. A 2017, 1524, 160–168.
- Kita, K.; Mano, Y. Application of volumetric absorptive microsampling device for quantification of tacrolimus in human blood as a model drug of high blood cell partition. J. Pharm. Biomed. Anal. 2017, 143, 168–175.
- Youhnovski, N.; Mayrand-Provencher, L.; Bérubé, E.R.; Plomley, J.; Montpetit, H.; Furtado, M.; Keyhani, A. Volumetric absorptive microsampling combined with impact-assisted extraction for hematocrit effect free assays. Bioanalysis 2017, 9, 1761–1769.
- Protti, M.; Catapano, M.C.; Samolsky Dekel, B.G.; Rudge, J.; Gerra, G.; Somaini, L.; Mandrioli, R.; Mercolini, L. Determination of oxycodone and its major metabolites in haematic and urinary matrices: Comparison of traditional and miniaturised sampling approaches. J. Pharm. Biomed. Anal. 2018, 152, 204–214.
- Kovač, J.; Panic, G.; Neodo, A.; Meister, I.; Coulibaly, J.T.; Schulz, J.D.; Keiser, J. Evaluation of a novel micro-sampling device, MitraTM, in comparison to dried blood spots, for analysis of praziquantel in Schistosoma haematobium-infected children in rural Côte d’Ivoire. J. Pharm. Biomed. Anal. 2018, 151, 339–346.
- Qu, Y.; Brady, K.; Apilado, R.; O’Malley, T.; Reddy, S.; Chitkara, P.; Ibarra, C.; Alexander, R.V.; Dervieux, T. Capillary blood collected on volumetric absorptive microsampling (VAMS) device for monitoring hydroxychloroquine in rheumatoid arthritis patients. J. Pharm. Biomed. Anal. 2017, 140, 334–341.
- Parker, S.L.; Roberts, J.A.; Lipman, J.; Wallis, S.C. Quantitative bioanalytical validation of fosfomycin in human whole blood with volumetric absorptive microsampling. Bioanalysis 2015, 7, 2585–2595.
- Houbart, V.; Cobraiville, G.; Servais, A.C.; Napp, A.; Merville, M.P.; Fillet, M. Hepcidin determination in dried blood by microfluidic LC-MS/MS: Comparison of DBS and volumetric absorptive microsampling for matrix effect and recovery. Bioanalysis 2015, 7, 2789–2799.
- Bolea-Fernandez, E.; Phan, K.; Balcaen, L.; Resano, M.; Vanhaecke, F. Determination of ultra-trace amounts of prosthesis-related metals in whole blood using volumetric absorptive micro-sampling and tandem ICP-Mass spectrometry. Anal. Chim. Acta 2016, 941, 1–9.
- Cañabate, Á.; García-Ruiz, E.; Resano, M.; Todolí, J.L. Analysis of whole blood by ICP-MS equipped with a high temperature total sample consumption system. J. Anal. At. Spectrom. 2017, 32, 78–87.
- John, H.; Willoh, S.; Hörmann, P.; Siegert, M.; Vondran, A.; Thiermann, H. Procedures for Analysis of Dried Plasma Using Microsampling Devices to Detect Sulfur Mustard-Albumin Adducts for Verification of Poisoning. Anal. Chem. 2016, 88, 8787–8794.
- Luo, Y.; Korfmacher, W.; Ho, S.; Shen, L.; Wang, J.; Wu, Z.; Guo, Y.; Snow, G.; O’shea, T. Evaluation of two blood microsampling approaches for drug discovery PK studies in rats. Bioanalysis 2015, 7, 2345–2359.
- Mercolini, L.; Protti, M.; Catapano, M.C.; Rudge, J.; Sberna, A.E. LC-MS/MS and volumetric absorptive microsampling for quantitative bioanalysis of cathinone analogues in dried urine, plasma and oral fluid samples. J. Pharm. Biomed. Anal. 2016, 123, 186–194.
- Miao, Z.; Farnham, J.G.; Hanson, G.; Podoll, T.; Reid, M.J. Bioanalysis of emixustat (ACU-4429) in whole blood collected with volumetric absorptive microsampling by LC-MS/MS. Bioanalysis 2015, 7, 2071–2083.
- Nys, G.; Gallez, A.; Kok, M.G.M.; Cobraiville, G.; Servais, A.C.; Piel, G.; Pequeux, C.; Fillet, M. Whole blood microsampling for the quantitation of estetrol without derivatization by liquid chromatography-tandem mass spectrometry. J. Pharm. Biomed. Anal. 2017, 140, 258–265.
- Thiry, J.; Evrard, B.; Nys, G.; Fillet, M.; Kok, M.G.M. Sampling only ten microliters of whole blood for the quantification of poorly soluble drugs: Itraconazole as case study. J. Chromatogr. A 2017, 1479, 161–168.
- Parker, S.L.; Guerra Valero, Y.C.; Lipman, J.; Roberts, J.A.; Wallis, S.C. Effect of time on recovery of plasma microsamples for the quantitative determination of vancomycin. Bioanalysis 2016, 8, 2235–2242.
- Londhe, V.; Rajadhyaksha, M. Opportunities and obstacles for microsampling techniques in bioanalysis: Special focus on DBS and VAMS. J. Pharm. Biomed. Anal. 2020, 182, 113102.
- Schulz, J.D.; Neodo, A.; Coulibaly, J.T.; Keiser, J. Pharmacokinetics of Albendazole, Albendazole Sulfoxide, and Albendazole Sulfone Determined from Plasma, Blood, Dried Blood Spots, and Mitra Samples of Hookworm-Infected Adolescents. Antimicrob. Agents Chemother. 2019, 63, e02489-18.
- Canisius, T.P.I.J.M.; Soons, J.W.P.H.; Verschuure, P.; Wammes-Van Der Heijden, E.A.; Rouhl, R.P.W.; Majoie, H.J.M. Therapeutic drug monitoring of anti-epileptic drugs—A clinical verification of volumetric absorptive micro sampling. Clin. Chem. Lab. Med. 2020, 58, 828–835.
- Tron, C.; Ferrand-Sorre, M.J.; Querzerho-Raguideau, J.; Chemouny, J.M.; Houssel-Debry, P.; Verdier, M.C.; Bellissant, E.; Lemaitre, F. Volumetric absorptive microsampling for the quantification of tacrolimus in capillary blood by high performance liquid chromatography-tandem mass spectrometry. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2021, 1165, 122521.
- Koster, R.A.; Niemeijer, P.; Veenhof, H.; van Hateren, K.; Alffenaar, J.-W.C.; Touw, D.J. A volumetric absorptive microsampling LC-MS/MS method for five immunosuppressants and their hematocrit effects. Bioanalysis 2019, 11, 495–508.
This entry is offline, you can click here to edit this entry!
