Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
The glymphatic system is a recently discovered waste clearance system that has been associated with many diseases including Alzheimer’s disease, hemorrhage, and neurotrauma. Thus, it opens an array of research opportunities to improve and understand their prognoses. Currently, ex vivo fluorescence microscopy of brain slices, MRI, and macroscopic cortical imaging are the most common ways of determining glymphatic system function.
- glymphatic system
- magnetic resonance imaging (MRI) sequencing
- imaging techniques
- neurological diseases
1. Introduction
Glymphatic flow is facilitated through the perivascular system and is an important feature of the glymphatic system. The use of diffusion MRI techniques can help map this flow and thereby broaden the understanding of the glymphatic system’s mechanism. This flow can then be further studied with diffusion tensor imaging (DTI), which can 3D map the flow pathway [1]. DTI can even evaluate patients with TBI, allowing for the quantification of the glymphatic pathway in both chronic- and acute-illness patients [2]. DTI shows the movement of water molecules and can be used to quantify the diffusivity of individuals, thus creating a better understanding of a patient’s glymphatic system [3].
Single-photon emission computed tomography/computed tomography (SPECT/CT) is another imaging technique that provides an understanding of the glymphatic drug delivery pathway. The kinetics of CSF tracer flow is limited in other imaging techniques due to their limited spatial detail. SPECT/CT imaging allows the quantification of a tracer’s clearance from the site of injection and enables the use of smaller doses of the tracer compared with contrast-enhanced MRI. This can then provide more knowledge about the impact of neurodegenerative diseases on glymphatic clearance [4].
Ultrafast magnetic resonance encephalography (MREG) can help image the entire brain in 100 ms and show the pulsation of CSF. By doing so, the glymphatic CSF flow pulsations can be better mapped and studied through MREG wave analysis. Mapping CSF flow will also help understand the decline of the glymphatic system and the onset of neurodegenerative diseases during the onset of aging [5].
Fluorescence microscopy and macroscopic imaging techniques are further imaging techniques that can help map the glymphatic system. The glymphatic system is highly active during the sleep state or under anesthesia when most waste clearance occurs while being suppressed during the awake state. Light sheet fluorescence microscopy can be used to detect these “sleep” and “awake” states of the glymphatic system. This imaging technique is particularly advantageous due to its ability to produce high-resolution imaging of the brain in a single session [6]. Additionally, transcranial glymphatic flow can be mapped with fluorescent macroscopy by delivering fluorescent tracers and using LED illumination for fluorophore excitation. In doing so, CSF flow in the glymphatic system can be further mapped [7].
2. Imaging Techniques
2.1. Glymphatic T1-Weighted MRI with Gadolinium Contrast
Gadolinium is a paramagnetic contrast agent that improves the visualization of CSF distribution in MRI. It is the most common contrast agent used in T1-weighted MRI to map the glymphatic pathway. Gadolinium-based contrast agents (GBCAs) can be administered both intravenously and intrathecally, with intravenous methods being well tolerated at a greater range of doses [8][9][10].
-
Intrathecal gadolinium enhancement of glymphatic T1 MRI:
Building off of their seminal work to discover the glymphatic system, Iliff et al. adapted MR cisternography techniques to successfully analyze the kinetics of CSF flow and layout of the glymphatic system in the rat brain. The team reported that intrathecal contrast agents move via bulk flow through several influx nodes along the surface and penetrate para-arterial pathways into the brain parenchyma. Glymphatic flux was observed to occur through bulk flow, independent of molecular weight, in the proximal perivascular channels and subarachnoid spaces. Conversely, the interstitial uptake of a small-molecular-weight GBCA, gadolinium-diethylenetriaminepentaacetate (Gd-DTPA), was more rapid compared with a large-molecular-weight polymeric gadolinium-chelate in the glymphatic system [11]. The human glymphatic pathway has also been mapped using GBCA in conjunction with T1 MRI. One study demonstrated brain-wide distribution and enhancement in serial T1-weighted MRI when gadobutrol was injected into the subarachnoid compartments of human subjects, noting a similar flow of the contrast agents to what was previously observed in the rodent brain [12]. Studies on the glymphatic system using MRI with intrathecal GBCAs have confirmed that the system is most active during sleep and that it is impaired in neurodegenerative diseases including Alzheimer’s disease and Parkinson’s disease [13][14]. It follows that MRI using GBCAs may one day be incorporated into screening criteria for sleep disorders and dementia.
- b.
-
Intravenous gadolinium enhancement of glymphatic T1 MRI:
The clinical usefulness of intrathecal gadolinium is limited by its invasiveness and the inherent risks of intrathecal injections. An alternative method, T1-weighted MRI with GBCAs administered intravenously, has been a topic of interest in recent years. Animal and human studies have thus far been targeted at understanding the path that GBCAs take from the blood to CSF in glymphatic circulation. In a study of six rats, intravenous injection of gadodiamide was found to coincide with an instantaneous increase in MRI signal intensity in the fourth ventricle, supporting the notion that GBCAs may immediately transfer from the blood to the CSF to reach the brain and glymphatic system [15]. A rapid change in T1 MRI values after the intravenous injection of a GBCA was also seen in a study of 25 healthy human subjects [16]. Taoka and Naganawa noted that GBCAs remain in glymphatic circulation significantly longer than they do in cerebral blood vessels, which was seen as a rapid decrease in the concentration of a GBCA due to renal elimination [17][18].
2.2. Diffusion Tensor Imaging/Diffusion MR Technique/4D Flow MRI
The dysregulation of the glymphatic system is also implicated in various neuroinflammatory and traumatic disease processes, including subdural hemorrhages and associated herniation syndromes [1][19]. Through diffusion MRI techniques, motion probing gradients (MPGs) that generate magnetic fields in various orientations can be used to assess the diffusion of glymphatic flow [1]. When these diffusion indices, denoted by the b value, are summated in 3D space, they are then visualized through diffusion tensor imaging (DTI) [1]. However, it should be noted that this technique solely maps the perivascular spaces (PVSs) and does not include extracranial drainage through the meninges or other routes.
There are several advantages to DTI over more traditional isotope tracer imaging modalities: While tracer studies have the limitation of relying on the tracer to spread temporally to distribute throughout the brain, DTI uses the magnetic spin of water molecules when MPGs are applied, providing a picture of the brain in real-time [1][20]. Similarly, DTI studies are also far less invasive, since tracer studies required tracer infusions administered intravenously or intrathecally.
In addition to DTI, four-dimensional flow-sensitive MRI (4D flow MRI) refers to phase-contrast MRI using blood flow in all three directions represented in a spatial and temporal fashion and can provide blood flow characteristics, including its direction and velocity [21]. In a study of 50 patients diagnosed with ischemic strokes, a higher acute lesion volume measured in DTI directly correlated with worsened clinical severity measured with the NIH Stroke Scale [22]. The ability of DTI to detect hyperacute ischemia in minutes means that DTI may be used in conjunction with perfusion studies to extrapolate clinical severity and prognosis in stroke management.
DTI is also useful for stroke management via the evaluation of CSF fluid in the perivascular space following a subarachnoid hemorrhage. This allows a clinician to predict the morbidity and damage following a stroke [23]. As discussed above, while both DTI and 4D flow MRI studies have great value in understanding the phenomenon of a dysfunctional glymphatic system following a hemorrhage, together, they are promising neuroimaging modalities that will be able to optimize the prognosticating and management of neurotrauma and inflammatory disorders in various clinical settings.
2.3. SPECT/CT Imaging
According to a recent study conducted in mice, it has been discovered that subarachnoid hemorrhages have a disruptive effect on the inflow of cerebrospinal fluid (CSF) after a 24 h period [24]. Fibrin and fibrinogen deposits occlude the perivascular spaces and result in the inhibition of the glymphatic system [25]. In traumatic brain injuries, mouse studies have found that there is a decrease in glymphatic influx along with diminished clearance of radiotracers, persisting for over 28 days following the injury [26].
Single-photon emission computed tomography/computed tomography (SPECT/CT) has historically been used to quantify brain perfusion while assessing receptor binding in numerous diseases, such as dementia, vascular diseases, cancers, and neuropsychiatric diseases [27][28]. SPECT imaging detects gamma rays and is used clinically and preclinically [29]. Combining SPECT with CT allows for the detection of tracer distribution to be visualized in three dimensions with a higher specificity and sensitivity than MRI [30].
2.4. Ultrafast MR Encephalography
Ultrafast magnetic resonance encephalography (MREG) can produce an image of the brain within 100 milliseconds [5]. Combining ultrafast MREG with cardiorespiratory monitoring allows for whole-brain coverage and allows for the analysis of all physiological pulsations [31]. Human studies using ultrafast MREG have found that there are three distinct pulsation mechanisms that maintain the flow of CSF and elimination of waste products within the brain: very low frequency (<0.1 Hz), respiratory (0.2–0.3 Hz), and cardiac (0.8–1.2 Hz) (Figure 1) [32]. The primary contributor to bulk CSF flow and exchange of CSF with interstitial fluid is believed to be arterial pulsations that occur during cardiac systole, allowing for solutes to move from the peri-arterial spaces into the extracellular brain tissue [33][34].
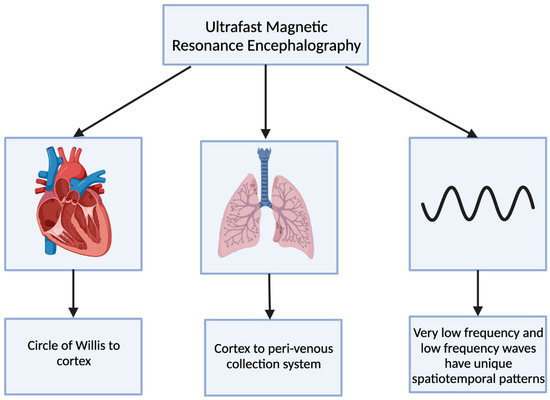
Figure 1. The 3 distinct pulsations of the glymphatic system captured using ultrafast magnetic resonance encephalography.
The cardiovascular pulsation creates a negative MREG signal impulse and then generates a positive charge within the cortex [5]. This pulsation begins at the basal peri-arterial spaces near the circle of Willis and then travels to the cortex [5]. Within the cortex, the respiratory pulsation is prominent and also extends into the peri-venous collection system [5]. The last pulsation is via the slow vasomotor waves seen via very low-frequency and low-frequency waves [5]. These slow vasomotor waves create unique spatiotemporal patterns [5].
In neurodegenerative diseases, impaired glymphatic clearance and drainage are believed to be dysfunctional; therefore, there is poor removal of protein debris [5]. Ultrafast MREG is capable of capturing glymphatic pulsation mechanisms and may be useful in elucidating the mechanisms resulting in glymphatic impairment and protein buildup in neurodegenerative conditions [35]. Ultrafast MREG may be useful in understanding the mechanism of glymphatic system dysfunction in neurodegenerative diseases as it can capture the distinct pulsations produced by the glymphatic system. However, further research is needed in which ultrafast MREG is used to map the glymphatic system in human and animal studies.
2.5. Fluorescence Microscopy
Fluorescence microscopy is a technique used to monitor cell physiology by making use of fluorescent agents to help increase the image contrast and spatial resolution of a given specimen [36]. These fluorescent molecules, known as fluorophores or fluorochromes, undergo photoluminescence, where portions of light are absorbed and emitted by the fluorophore. These signals of light are detected with a camera system that allows for enhanced imaging under the microscope [37].
The glymphatic system has been associated with diseases which are caused by the accumulation of certain compounds. Utilizing fluorophores to attach to pathologic solutes of interest can allow the visualization of these solutes and their presence in and clearance from the glymphatic system [38]. Utilization of this technique was demonstrated in one study regarding Alzheimer’s disease in mice. The visualization of amyloid plaques in the brains of mice demonstrated a reduction in glymphatic influx and clearance of amyloid plaques as the mice aged, displaying how the concentration and clearance of proteins within the brain can be observed [26].
Fluorophores were also used in mice to map the glymphatic system by injecting the mice with fluorescently labeled dextrans. The mice dextrans were injected into the cerebrospinal fluid and were visualized traveling into the perivascular spaces of surface arteries and then moving deeper into penetrating arteries. Furthermore, in Tie2-GFP:NG2-DsRed double reporter mice, the fluorescent tracer i.e., the ovalbumin passed through the astrocyte foot-processes and glial-limiting membranes by entering through the perivascular space between the smooth muscle cells. Following an injection of fluorescent tracer, the arteries, veins, capillaries were able to be directly identified [39]. The use of fluorescent tracers validated the perivascular influx of CSF and the directionality of this fluid movement with CSF entering the brain exclusively via periarterial spaces and ISF leaving the brain via perivenous channels [26][39].
Two-photon fluorescence laser-scanning microscopy, a variation of fluorescence microscopy, is a technique that can facilitate live high-resolution spatiotemporal imaging. Two-photon fluorescence laser-scanning microscopy can be used to obtain the visualization of the whole-cortex vascular 3D structure, as well as to understand the blood flow in the capillary network of the brain. It is also useful in measuring the size, speed, and flux of structures within the brain and the cranial window. This imaging technique allows for a deeper understanding of space and time regarding the images taken, and thus providing direct evidence regarding the pathophysiology uncovering after a neurotrauma such as an ischemic stroke [38][40].
Fluorescent microscopy provides powerful advanced imaging, allows for real-time evaluation of the presence and flow of desired molecules through the brain, and allows for multidimensional analysis of brain structure and pathophysiological mechanisms of neurotrauma and disease [40][41][42][43][44].
2.6. Macroscopic Imaging Techniques
Macroscopic imaging techniques include the utilization of macroscopic CSF tracers, transcranial macroscopic imaging, and fluorescence macroscopy. These imaging techniques utilize the use of fluorophores to bind to compounds of interest and provide quantitative information on compound concentrations and distribution through optically turbid media, such as tissue (Figure 2). The use of this technology allows for the possibility to study and analyze intact organ structures and provides information about how therapies can be more optimally administered to reach their target destination, and it can be used for guidance during operations such as brain tumor resections [44][45].
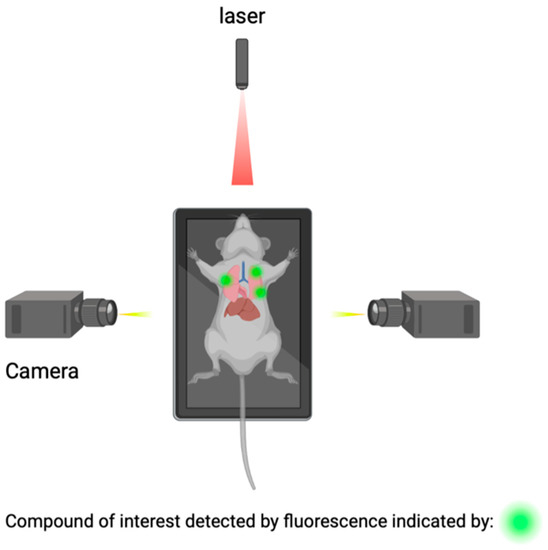
Figure 2. Detection of compounds of interest through the utilization of fluorophores.
Transcranial macroscopic imaging has been used to noninvasively evaluate the in vivo delivery pathways of CSF fluorescent tracers that were shown to be distributed through the glymphatic system. Transcranial macroscopic imaging was able to determine that CSF tracer entry increased by three-fold without disruption of the BBB when the plasma osmolality was increased. Additionally in a mouse model, it showed that increasing the plasma osmolality reversed the inhibited glymphatic flow and suppression, which is a characteristic of the awake state of Alzheimer’s disease. Lastly, it showed that an increased plasma osmolality also showed a five-fold increase in the binding of antibodies to amyloid-β (Aβ) plaques, via enhancing its delivery. The utilization of a combination of macroscopic CSF tracers and transcranial macroscopic imaging spearheaded the possibility to visualize and study how to improve the penetration of therapeutic antibodies to the CNS [7][46][47][48][49].
Real-time image-guided surgical tumor excisions promise to improve clinical outcomes and prolong the lives of patients. Histopathological analysis of rat brains ex vivo showed the location and invasion of the tumor within the deep structures of the brain [45][50]. Characteristics of tumors such as cell type and degree of vascularization can also be observed and studied [45][50]. Advanced-stage glioma is the most aggressive form of malignant brain tumor with a short survival time [51]. A study evaluating time-resolved laser-induced fluorescence spectroscopy was able to classify low-grade gliomas with 100% sensitivity and 98% specificity. This provides a potentially valuable tool for neurosurgeon neuropathology teams to rapidly distinguish between tumor and normal brain tissue during surgery [52].
This entry is adapted from the peer-reviewed paper 10.3390/j6030031
References
- Taoka, T.; Naganawa, S. Glymphatic imaging using MRI. J. Magn. Reason. Imaging 2020, 51, 11–24.
- Park, J.H.; Bae, Y.J.; Kim, J.S.; Jung, W.S.; Choi, J.W.; Roh, T.H.; You, N.; Kim, S.H.; Han, M. Glymphatic system evaluation using diffusion tensor imaging in patients with traumatic brain injury. Neuroradiology 2023, 65, 551–557.
- Klostranec, J.M.; Vucevic, D.; Bhatia, K.D.; Kortman, H.G.J.; Krings, T.; Murphy, K.P.; terBrugge, K.G.; Mikulis, D.J. Current Concepts in Intracranial Interstitial Fluid Transport and the Glymphatic System: Part II-Imaging Techniques and Clinical Applications. Radiology 2021, 301, 516–532.
- Lilius, T.O.; Rosenholm, M.; Klinger, L.; Mortensen, K.N.; Sigurdsson, B.; Mogensen, F.L.; Hauglund, N.L.; Nielsen, M.S.N.; Rantamäki, T.; Nedergaard, M. SPECT/CT imaging reveals CNS-wide modulation of glymphatic cerebrospinal fluid flow by systemic hypertonic saline. iScience 2022, 25, 105250.
- Kiviniemi, V.; Wang, X.; Korhonen, V.; Keinänen, T.; Tuovinen, T.; Autio, J.; LeVan, P.; Keilholz, S.; Zang, Y.F.; Hennig, J.; et al. Ultra-fast magnetic resonance encephalography of physiological brain activity-Glymphatic pulsation mechanisms? J. Cereb. Blood Flow Metab. 2016, 36, 1033–1045.
- Bèchet, N.B.; Kylkilahti, T.M.; Mattsson, B.; Petrasova, M.; Shanbhag, N.C.; Lundgaard, I. Light sheet fluorescence microscopy of optically cleared brains for studying the glymphatic system. J. Cereb. Blood Flow Metab. 2020, 40, 1975–1986.
- Plog, B.A.; Mestre, H.; Olveda, G.E.; Sweeney, A.M.; Kenney, H.M.; Cove, A.; Dholakia, K.Y.; Tithof, J.; Nevins, T.D.; Lundgaard, I.; et al. Transcranial optical imaging reveals a pathway for optimizing the delivery of immunotherapeutics to the brain. JCI Insight 2018, 3, 120922.
- Arlt, S.; Cepek, L.; Rustenbeck, H.H.; Prange, H.; Reimers, C.D. Gadolinium encephalopathy due to accidental intrathecal administration of gadopentetate dimeglumine. J. Neurol. 2007, 254, 810–812.
- Reeves, C.; Galang, E.; Padalia, R.; Tran, N.; Padalia, D. Intrathecal Injection of Gadobutrol: A Tale of Caution. J. Pain. Palliat. Care Pharmacother. 2017, 31, 139–143.
- Edeklev, C.S.; Halvorsen, M.; Løvland, G.; Vatnehol, S.A.S.; Gjertsen, Ø.; Nedregaard, B.; Sletteberg, R.; Ringstad, G.; Eide, P.K. Intrathecal Use of Gadobutrol for Glymphatic MR Imaging: Prospective Safety Study of 100 Patients. AJNR Am. J. Neuroradiol. 2019, 40, 1257–1264.
- Iliff, J.J.; Lee, H.; Yu, M.; Feng, T.; Logan, J.; Nedergaard, M.; Benveniste, H. Brain-wide pathway for waste clearance captured by contrast-enhanced MRI. J. Clin. Investig. 2013, 123, 1299–1309.
- Ringstad, G.; Valnes, L.M.; Dale, A.M.; Pripp, A.H.; Vatnehol, S.S.; Emblem, K.E.; Mardal, K.A.; Eide, P.K. Brain-wide glymphatic enhancement and clearance in humans assessed with MRI. JCI Insight 2018, 3, 121537.
- Lee, H.; Choi, S.H.; Anzai, Y. Glymphatic MRI techniques in sleep and neurodegenerative diseases. Curr. Opin. Pulm. Med. 2022, 28, 499–510.
- Petiet, A.; Santin, M.; Bertrand, A.; Wiggins, C.J.; Petit, F.; Houitte, D.; Hantraye, P.; Benavides, J.; Debeir, T.; Rooney, T.; et al. Gadolinium-staining reveals amyloid plaques in the brain of Alzheimer’s transgenic mice. Neurobiol. Aging 2012, 33, 1533–1544.
- Taoka, T.; Jost, G.; Frenzel, T.; Naganawa, S.; Pietsch, H. Impact of the Glymphatic System on the Kinetic and Distribution of Gadodiamide in the Rat Brain: Observations by Dynamic MRI and Effect of Circadian Rhythm on Tissue Gadolinium Concentrations. Investig. Radiol. 2018, 53, 529–534.
- Lee, S.; Yoo, R.E.; Choi, S.H.; Oh, S.H.; Ji, S.; Lee, J.; Huh, K.Y.; Lee, J.Y.; Hwang, I.; Kang, K.M.; et al. Contrast-enhanced MRI T1 Mapping for Quantitative Evaluation of Putative Dynamic Glymphatic Activity in the Human Brain in Sleep-Wake States. Radiology 2021, 300, 661–668.
- Taoka, T.; Naganawa, S. Gadolinium-based Contrast Media, Cerebrospinal Fluid and the Glymphatic System: Possible Mechanisms for the Deposition of Gadolinium in the Brain. Magn. Reason. Med. Sci. 2018, 17, 111–119.
- Richmond, S.B.; Rane, S.; Hanson, M.R.; Albayram, M.; Iliff, J.J.; Kernagis, D.; Rosenberg, J.T.; Seidler, R.D. Quantification approaches for magnetic resonance imaging following intravenous gadolinium injection: A window into brain-wide glymphatic function. Eur. J. Neurosci. 2023, 57, 1689–1704.
- Iliff, J.J.; Nedergaard, M. Is there a cerebral lymphatic system? Stroke 2013, 44 (Suppl. 1), S93–S95.
- Luo, C.; Yao, X.; Li, J.; He, B.; Liu, Q.; Ren, H.; Liang, F.; Li, M.; Lin, H.; Peng, J.; et al. Paravascular pathways contribute to vasculitis and neuroinflammation after subarachnoid hemorrhage independently of glymphatic control. Cell Death Dis. 2016, 7, e2160.
- Zhuang, B.; Sirajuddin, A.; Zhao, S.; Lu, M. The role of 4D flow MRI for clinical applications in cardiovascular disease: Current status and future perspectives. Quant. Imaging Med. Surg. 2021, 11, 4193–4210.
- Lövblad, K.O.; Baird, A.E.; Schlaug, G.; Benfield, A.; Siewert, B.; Voetsch, B.; Connor, A.; Burzynski, C.; Edelman, R.R.; Warach, S. Ischemic lesion volumes in acute stroke by diffusion-weighted magnetic resonance imaging correlate with clinical outcome. Ann. Neurol. 1997, 42, 164–170.
- Taoka, T.; Masutani, Y.; Kawai, H.; Nakane, T.; Matsuoka, K.; Yasuno, F.; Kishimoto, T.; Naganawa, S. Evaluation of glymphatic system activity with the diffusion MR technique: Diffusion tensor image analysis along the perivascular space (DTI-ALPS) in Alzheimer’s disease cases. Jpn. J. Radiol. 2017, 35, 172–178.
- Gaberel, T.; Gakuba, C.; Goulay, R.; De Lizarrondo, S.M.; Hanouz, J.L.; Emery, E.; Touze, E.; Vivien, D.; Gauberti, M. Impaired glymphatic perfusion after strokes revealed by contrast-enhanced MRI: A new target for fibrinolysis? Stroke 2014, 45, 3092–3096.
- Goulay, R.; Flament, J.; Gauberti, M.; Naveau, M.; Pasquet, N.; Gakuba, C.; Emery, E.; Hantraye, P.; Vivien, D.; Aron-Badin, R.; et al. Subarachnoid Hemorrhage Severely Impairs Brain Parenchymal Cerebrospinal Fluid Circulation in Nonhuman Primate. Stroke 2017, 48, 2301–2305.
- Iliff, J.J.; Chen, M.J.; Plog, B.A.; Zeppenfeld, D.M.; Soltero, M.; Yang, L.; Singh, I.; Deane, R.; Nedergaard, M. Impairment of glymphatic pathway function promotes tau pathology after traumatic brain injury. J. Neurosci. 2014, 34, 16180–16193.
- Valotassiou, V.; Malamitsi, J.; Papatriantafyllou, J.; Dardiotis, E.; Tsougos, I.; Psimadas, D.; Alexiou, S.; Hadjigeorgiou, G.; Georgoulias, P. SPECT and PET imaging in Alzheimer’s disease. Ann. Nucl. Med. 2018, 32, 583–593.
- van der Vaart, M.G.; Meerwaldt, R.; Slart, R.H.; van Dam, G.M.; Tio, R.A.; Zeebregts, C.J. Application of PET/SPECT imaging in vascular disease. Eur. J. Vasc. Endovasc. Surg. 2008, 35, 507–513.
- Goffin, K.; van Laere, K. Single-photon emission tomography. Handb. Clin. Neurol. 2016, 135, 241–250.
- Sigurdsson, B.; Hauglund, N.L.; Lilius, T.O.; Mogensen, F.L.; Mortensen, K.N.; Beschorner, N.; Klinger, L.; Bærentzen, S.L.; Rosenholm, M.P.; Shalgunov, V.; et al. A SPECT-based method for dynamic imaging of the glymphatic system in rats. J. Cereb. Blood Flow Metab. 2023, 43, 1153–1165.
- Korhonen, V.; Hiltunen, T.; Myllylä, T.; Wang, X.; Kantola, J.; Nikkinen, J.; Zang, Y.F.; LeVan, P.; Kiviniemi, V. Synchronous multiscale neuroimaging environment for critically sampled physiological analysis of brain function: Hepta-scan concept. Brain Connect 2014, 4, 677–689.
- Kaur, J.; Davoodi-Bojd, E.; Fahmy, L.M.; Zhang, L.; Ding, G.; Hu, J.; Zhang, Z.; Chopp, M.; Jiang, Q. Magnetic Resonance Imaging and Modeling of the Glymphatic System. Diagnostics 2020, 10, 344.
- Iliff, J.J.; Wang, M.; Zeppenfeld, D.M.; Venkataraman, A.; Plog, B.A.; Liao, Y.; Deane, R.; Nedergaard, M. Cerebral arterial pulsation drives paravascular CSF-interstitial fluid exchange in the murine brain. J. Neurosci. 2013, 33, 18190–18199.
- Mestre, H.; Tithof, J.; Du, T.; Song, W.; Peng, W.; Sweeney, A.M.; Olveda, G.; Thomas, J.H.; Nedergaard, M.; Kelley, D.H. Flow of cerebrospinal fluid is driven by arterial pulsations and is reduced in hypertension. Nat. Commun. 2018, 9, 4878.
- Iliff, J.J.; Wang, M.; Liao, Y.; Plogg, B.A.; Peng, W.; Gundersen, G.A.; Benveniste, H.; Vates, G.E.; Deane, R.; Goldman, S.A.; et al. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid β. Sci. Transl. Med. 2012, 4, 147ra111.
- Sanderson, M.J.; Smith, I.; Parker, I.; Bootman, M.D. Fluorescence microscopy. Cold Spring Harb. Protoc. 2014, 2014, pdb.top071795.
- Plog, B.A.; Nedergaard, M. The Glymphatic System in Central Nervous System Health and Disease: Past, Present, and Future. Annu. Rev. Pathol. 2018, 13, 379–394.
- Dunst, S.; Tomancak, P. Imaging Flies by Fluorescence Microscopy: Principles, Technologies, and Applications. Genetics 2019, 211, 15–34.
- Wu, X.; Li, J.R.; Fu, Y.; Chen, D.Y.; Nie, H.; Tang, Z.P. From static to dynamic: Live observation of the support system after ischemic stroke by two photon-excited fluorescence laser-scanning microscopy. Neural. Regen. Res. 2023, 18, 2093–2107.
- Li, J.; Wu, X.; Fu, Y.; Nie, H.; Tang, Z. Two-photon microscopy: Application advantages and latest progress for. Rev. Neurosci. 2023, 34, 559–572.
- Helmchen, F.; Kleinfeld, D. Chapter 10. In vivo measurements of blood flow and glial cell function with two-photon laser-scanning microscopy. Methods Enzymol. 2008, 444, 231–254.
- De, A.K.; Goswami, D. Adding new dimensions to laser-scanning fluorescence microscopy. J. Microsc. 2009, 233, 320–325.
- MacRitchie, N.; Maffia, P. Light sheet fluorescence microscopy for quantitative three-dimensional imaging of vascular remodelling. Cardiovasc. Res. 2021, 117, 348–350.
- Jermyn, M.; Kolste, K.; Pichette, J.; Sheehy, G.; Angulo-Rodríguez, L.; Paulsen, K.D.; Roberts, D.W.; Wilson, B.C.; Petrecca, K.; Leblond, F. Macroscopic-imaging technique for subsurface quantification of near-infrared markers during surgery. J. Biomed. Opt. 2015, 20, 036014.
- Lukina, M.; Yashin, K.; Kiseleva, E.E.; Alekseeva, A.; Dudenkova, V.; Zagaynova, E.V.; Bederina, E.; Medyanic, I.; Becker, W.; Mishra, D.; et al. Label-Free Macroscopic Fluorescence Lifetime Imaging of Brain Tumors. Front. Oncol. 2021, 11, 666059.
- Yuan, Y.; Yan, Z.; Miao, J.; Cai, R.; Zhang, M.; Wang, Y.; Wang, L.; Dang, W.; Wang, D.; Xiang, D.; et al. Autofluorescence of NADH is a new biomarker for sorting and characterizing cancer stem cells in human glioma. Stem. Cell Res. Ther. 2019, 10, 330.
- Shcheslavskiy, V.I.; Shirmanova, M.V.; Dudenkova, V.V.; Lukyanov, K.A.; Gavrina, A.I.; Shumilova, A.V.; Zagaynova, E.; Becker, W. Fluorescence time-resolved macroimaging. Opt. Lett. 2018, 43, 3152–3155.
- Erkkilä, M.T.; Reichert, D.; Gesperger, J.; Kiesel, B.; Roetzer, T.; Mercea, P.A.; Drexler, W.; Unterhuber, A.; Leitgeb, R.A.; Woehrer, A.; et al. Macroscopic fluorescence-lifetime imaging of NADH and protoporphyrin IX improves the detection and grading of 5-aminolevulinic acid-stained brain tumors. Sci. Rep. 2020, 10, 20492.
- Harrison, I.F.; Ismail, O.; Machhada, A.; Colgan, N.; Ohene, Y.; Nahavandi, P.; Ahmed, Z.; Fisher, A.; Meftah, S.; Murray, T.K.; et al. Impaired glymphatic function and clearance of tau in an Alzheimer’s disease model. Brain 2020, 143, 2576–2593.
- Wagnières, G.A.; Star, W.M.; Wilson, B.C. In vivo fluorescence spectroscopy and imaging for oncological applications. Photochem. Photobiol. 1998, 68, 603–632.
- Stupp, R.; Mason, W.P.; van den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 2005, 352, 987–996.
- Butte, P.V.; Mamelak, A.N.; Nuno, M.; Bannykh, S.I.; Black, K.L.; Marcu, L. Fluorescence lifetime spectroscopy for guided therapy of brain tumors. Neuroimage 2011, 54, S125–S135.
This entry is offline, you can click here to edit this entry!
