Bovine mastitis is a polymicrobial disease characterised by inflammation of the udders of dairy and beef cattle. The infection has huge implications to health and welfare of animals, impacting milk and beef production and costing up to EUR 32 billion annually to the dairy industry, globally. Bacterial communities associated with the disease include representative species from Staphylococcus, Streptococcus, Enterococcus, Actinomyces, Aerococcus, Escherichia, Klebsiella and Proteus. Conventional treatment relies on antibiotics, but antimicrobial resistance, declining antibiotic innovations and biofilm production negatively impact therapeutic efficacy. Bacteriophages (phages) are viruses which effectively target and lyse bacteria with extreme specificity and can be a valuable supplement or replacement to antibiotics for bovine mastitis.
- Staphylococcus
- Streptococcus
- Enterococcus
- Actinomyces
- Aerococcus
1. Impact of Bovine Mastitis
Although the main cause of mastitis is bacterial sources, this is not because of any one particular species as there are a number of different species which can cause the condition. The major organisms (represented by >1% of all Isolates) identified in the work of Aarestrup et al. were generally members of the genus Staphylococcus, most commonly S. aureus, S. chromogenes, S. epidermidis, S. haemolyticus, S. simulans, and S. warneri, together with organisms from other genera, namely Streptococcus dysgalactiae, S. uberis, S. canis and Enterococcus faecalis [5]. However, the researchers also succeeded in isolating other members of the genus Staphylococcus at lower frequencies, namely S. auricularis, S. capitis, S. cohnii, S. hominis, S. lentis, S. muscae, S. saprophyticus and S. sciuru, together with non-specified/unidentified members of the same genus. The other sources of infection which they isolated were Actinomyces pyogenes, Aerococcus hydro, A. viridans, E. avium, E. durans, E. faecium, Escherichia coli, Klebsiella pneumoniae, unidentified members of the genus Proteus, S. lactis and S. salivarius. However, this is just an example of a single case study, and other studies have reported cases where some of the organisms found at minor levels were either present at higher relative abundance or as the sole or predominant causal organism, or indeed found other organisms capable of causing mastitis [6].
An example of another organism with the potential to cause mastitis is S. agalactiae. This is an organism which has been isolated from a range of different animals, including non-mammalian species [7], demonstrating that its role goes beyond causing mastitis. The example of S. agalactiae is an interesting one as it is not only able to infect a range of host species, but it can also be the causal organism for more than one clinical condition. For example, as well as being an organism which can lead to bovine mastitis, it has been described as being responsible for both sepsis and meningitis in humans, and also meningoencephalitis in fish [7]. Moreover, even at subclinical levels, S. agalactiae has been shown to have a detrimental impact on milk production, in terms of both quantity and milk quality [8]. Although a fairly large number of species are included in the list above, there are other examples of species which have been associated with the onset of mastitis. These include K. oxytoca [9], Mycobacterium bovis [10], Pseudomonas aeruginosa [11] and S. xylosus [12,13].
The obvious multidrug resistance shown towards a wide range of chemical antimicrobials are compelling and triggers the urgent need to identify and develop alternative strategies to control bovine mastitis safely and effectively [19,20]. A novel and emerging treatment which explores the application of bacteriophages (phages, viruses of bacteria) has been shown to greatly mitigate bacterial resistance and can improve the general health and production capacity of livestock [20,26].
2. The Case for Phage Therapy to Control Bovine Mastitis
2.1. Phage Specificity, Lysis and Amplification
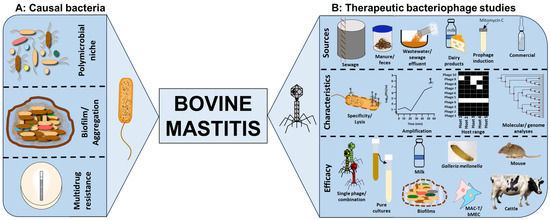
2.2. Isolation of Phages from a Wide Range of Sources
2.3. Cocktail Optimisation to Improve Therapeutic Activity
2.4. Characterisation of Phage Lysis and Stability in Pure Cultures
2.5. Phage Therapeutic Activity in Biofilms
2.6. Phage Therapeutic Assessments in Mastitis Ex Vivo and In Vivo Models
3. Barriers/Challenges to Therapeutic Phage Application to Control Bovine Mastitis
This entry is adapted from the peer-reviewed paper 10.3390/antibiotics12081307
References
- Bond, D.M.; Morris, J.M.; Nassar, N. Study protocol: Evaluation of the probiotic Lactobacillus Fermentum CECT5716 for the prevention of mastitis in breastfeeding women: A randomised controlled trial. BMC Pregnancy Childbirth 2017, 17, 148.
- Radostits, O.M.; Gay, C.C.; Hinchcliff, K.W.; Constable, P.D. Veterinary Medicine: A Textbook of the Diseases of Cattle, Horses, Sheep, Pigs and Goats, 10th ed.; Elsevier: St. Louis, MO, USA, 2007.
- Persson Waller, K.; Persson, Y.; Nyman, A.K.; Stengärde, L. Udder health in beef cows and its association with calf growth. Acta Vet. Scand. 2014, 56, 9.
- Rollin, E.; Dhuyvetter, K.C.; Overton, M.W. The cost of clinical mastitis in the first 30 days of lactation: An economic modeling tool. Prev. Vet. Med. 2015, 122, 257–264.
- Loc-Carrillo, C.; Abedon, S.T. Pros and cons of phage therapy. Bacteriophage 2011, 1, 111–114.
- Taslem Mourosi, J.; Awe, A.; Guo, W.; Batra, H.; Ganesh, H.; Wu, X.; Zhu, J. Understanding Bacteriophage Tail Fiber Interaction with Host Surface Receptor: The Key “Blueprint” for Reprogramming Phage Host Range. Int. J. Mol. Sci. 2022, 23, 12146.
- Kizziah, J.L.; Manning, K.A.; Dearborn, A.D.; Dokland, T. Structure of the host cell recognition and penetration machinery of a Staphylococcus aureus bacteriophage. PLoS Pathog. 2020, 16, e1008314.
- Leite, J.A.; Pereira, H.P.; Borges, C.A.V.; Alves, B.R.C.; Ramos, A.; Martins, M.F.; Arcuri, E.F. Lytic bacteriophages as a potential alternative to control Staphylococcus aureus. Pesqui. Agropecu. Bras. 2019, 54.
- Bai, Q.; Zhang, W.; Yang, Y.; Tang, F.; Nguyen, X.; Liu, G.; Lu, C. Characterization and genome sequencing of a novel bacteriophage infecting Streptococcus agalactiae with high similarity to a phage from Streptococcus pyogenes. Arch. Virol. 2013, 158, 1733–1741.
- Wang, Z.F.; Zheng, P.P.; Ji, W.H.; Fu, Q.; Wang, H.G.; Yan, Y.X.; Sun, J.H. SLPW: A Virulent Bacteriophage Targeting Methicillin-Resistant Staphylococcus aureus In vitro and In vivo. Front. Microbiol. 2016, 7, 934.
- Wang, Z.; Xue, Y.; Gao, Y.; Guo, M.; Liu, Y.; Zou, X.; Cheng, Y.; Ma, J.; Wang, H.; Sun, J.; et al. Phage vB_PaeS-PAJD-1 Rescues Murine Mastitis Infected With Multidrug-Resistant Pseudomonas aeruginosa. Front. Cell Infect. Microbiol. 2021, 11, 689770.
- Zhang, Q.; Yu, H.; Sun, Y.; Zhangxiang, L.; Zhang, P.; Liu, G.; Qu, Y.; Tong, Y.; Li, Y. Isolation and characterization of a lytic phage infecting Enterococcus faecium of bovine mastitis. Acta Vet. Zootech. Sin. 2017, 48, 706–713.
- Wang, I.N.; Smith, D.L.; Young, R. Holins: The protein clocks of bacteriophage infections. Annu. Rev. Microbiol. 2000, 54, 799–825.
- Han, J.E.; Kim, J.H.; Hwang, S.Y.; Choresca, C.H., Jr.; Shin, S.P.; Jun, J.W.; Chai, J.Y.; Park, Y.H.; Park, S.C. Isolation and characterization of a Myoviridae bacteriophage against Staphylococcus aureus isolated from dairy cows with mastitis. Res. Vet. Sci. 2013, 95, 758–763.
- Makumi, A.; Mhone, A.L.; Odaba, J.; Guantai, L.; Svitek, N. Phages for Africa: The Potential Benefit and Challenges of Phage Therapy for the Livestock Sector in Sub-Saharan Africa. Antibiotics 2021, 10, 1085.
- Ferriol-González, C.; Domingo-Calap, P. Phage Therapy in Livestock and Companion Animals. Antibiotics 2021, 10, 559.
- Xi, H.Y.; He, D.L.; Li, D.; Liu, S.S.; Wang, G.; Ji, Y.L.; Wang, X.W.; Wang, Z.J.; Bi, L.T.; Zhao, R.H.; et al. Bacteriophage Protects Against Aerococcus viridans Infection in a Murine Mastitis Model. Front. Vet. Sci. 2020, 7, 588.
- Duarte, V.D.; Dias, R.S.; Kropinski, A.M.; Campanaro, S.; Treu, L.; Siqueira, C.; Vieira, M.S.; Paes, I.D.; Santana, G.R.; Martins, F.; et al. Genomic analysis and immune response in a murine mastitis model of vB_EcoM-UFV13, a potential biocontrol agent for use in dairy cows. Sci. Rep. 2018, 8, 6845.
- Fahliyani, S.A.; Beheshti-Maal, K.; Ghandehari, F. Novel lytic bacteriophages of Klebsiella oxytoca ABG-IAUF-1 as the potential agents for mastitis phage therapy. Fems Microbiol. Lett. 2018, 365, fny223.
- Hendrix, R.W.; Smith, M.C.M.; Burns, R.N.; Ford, M.E.; Hatfull, G.F. Evolutionary relationships among diverse bacteriophages and prophages: All the world’s a phage. Proc. Natl. Acad. Sci. USA 1999, 96, 2192–2197.
- Mushegian, A.R. Are There 1031 Virus Particles on Earth, or More, or Fewer? J. Bacteriol. 2020, 202, 10–1128.
- Clokie, M.R.; Millard, A.D.; Letarov, A.V.; Heaphy, S. Phages in nature. Bacteriophage 2011, 1, 31–45.
- Barasuol, B.M.; Cargnelutti, J.F.; Sangioni, L.A.; Pereira, D.I.B.; Varela, A.P.M.; Mayer, F.Q.; Pottker, E.S.; Goncalves, G.F.; Cibulski, S.; Botton, S.D. Characterization of novel of temperate phages of Staphylococcus aureus isolated from bovine milk. Arch. Microbiol. 2022, 204, 680.
- García, P.; Madera, C.; Martínez, B.; Rodríguez, A.; Evaristo Suárez, J. Prevalence of bacteriophages infecting Staphylococcus aureus in dairy samples and their potential as biocontrol agents. J. Dairy Sci. 2009, 92, 3019–3026.
- Basdew, I.H.; Laing, M.D. Investigation of the lytic ability of South African bacteriophages specific for Staphylococcus aureus, associated with bovine mastitis. Biocontrol Sci. Technol. 2015, 25, 429–443.
- Jia, H.; Bai, Q.; Yang, Y.; Yao, H. Complete Genome Sequence of Staphylococcus aureus Siphovirus Phage JS01. Genome Announc. 2013, 1, e00797-13.
- Jia, H.; Dong, W.; Yuan, L.; Ma, J.; Bai, Q.; Pan, Z.; Lu, C.; Yao, H. Characterization and complete genome sequence analysis of Staphylococcus aureus bacteriophage JS01. Virus Genes 2015, 50, 345–348.
- Slanetz, L.W.; Jawetz, E. Isolation and Characteristics of Bacteriophages for Staphylococci of Bovine Mastitis. J. Bacteriol. 1941, 41, 447–455.
- Teng, F.; Xiong, X.; Zhang, S.; Li, G.; Wang, R.; Zhang, L.; Wang, X.; Zhou, H.; Li, J.; Li, Y.; et al. Efficacy Assessment of Phage Therapy in Treating Staphylococcus aureus-Induced Mastitis in Mice. Viruses 2022, 14, 620.
- Han, G.; Zhang, J.; Luo, Z.; Lu, B.; Zhang, P.; Yong, K.; Wang, Y.; Luo, Y.; Yang, Z.; Ren, M.; et al. Characteristics of a novel temperate bacteriophage against Staphylococcus arlettae (vB_SarS_BM31). Int. Microbiol. 2023, 26, 327–341.
- Srujana, A.S.; Sonalika, J.; Akhila, D.S.; Juliet, M.R.; Sheela, P. Isolation of Phages and Study of their In vitro Efficacy on Staphylococcus aureus Isolates Originating from Bovine Subclinical Mastitis. Indian. J. Anim. Res. 2022, 56, 754–758.
- Gill, J.J.; Sabour, P.M.; Leslie, K.E.; Griffiths, M.W. Bovine whey proteins inhibit the interaction of Staphylococcus aureus and bacteriophage K. J. Appl. Microbiol. 2006, 101, 377–386.
- Bai, Q.; Yang, Y.; Lu, C. Isolation and characterization of siphovirus phages infecting bovine Streptococcus agalactiae. Wei Sheng Wu Xue Bao 2016, 56, 317–326.
- Dedrick, R.M.; Guerrero-Bustamante, C.A.; Garlena, R.A.; Russell, D.A.; Ford, K.; Harris, K.; Gilmour, K.C.; Soothill, J.; Jacobs-Sera, D.; Schooley, R.T.; et al. Engineered bacteriophages for treatment of a patient with a disseminated drug-resistant Mycobacterium abscessus. Nat. Med. 2019, 25, 730–733.
- Nale, J.Y.; Clokie, M.R.J. Preclinical data and safety assessment of phage therapy in humans. Curr. Opin. Biotechnol. 2021, 68, 310–317.
- Dias, R.S.; Eller, M.R.; Duarte, V.S.; Pereira, Â.L.; Silva, C.C.; Mantovani, H.C.; Oliveira, L.L.; Silva Ede, A.; De Paula, S.O. Use of phages against antibiotic-resistant Staphylococcus aureus isolated from bovine mastitis. J. Anim. Sci. 2013, 91, 3930–3939.
- Iwano, H.; Inoue, Y.; Takasago, T.; Kobayashi, H.; Furusawa, T.; Taniguchi, K.; Fujiki, J.; Yokota, H.; Usui, M.; Tanji, Y.; et al. Bacteriophage Phi SA012 Has a Broad Host Range against Staphylococcus aureus and Effective Lytic Capacity in a Mouse Mastitis Model. Biology 2018, 7, 8.
- Li, L.P.; Zhang, Z.Y. Isolation and characterization of a virulent bacteriophage SPW specific for Staphylococcus aureus isolated from bovine mastitis of lactating dairy cattle. Mol. Biol. Rep. 2014, 41, 5829–5838.
- Zhang, L.; Bao, H.; Wei, C.; Zhang, H.; Zhou, Y.; Wang, R. Characterization and partial genomic analysis of a lytic Myoviridae bacteriophage against Staphylococcus aureus isolated from dairy cows with mastitis in Mid-east of China. Virus Genes 2015, 50, 111–117.
- Zhang, L.L.; Sun, L.C.; Wei, R.C.; Gao, Q.; He, T.; Xu, C.F.; Liu, X.J.; Wang, R. Intracellular Staphylococcus aureus Control by Virulent Bacteriophages within MAC-T Bovine Mammary Epithelial Cells. Antimicrob. Agents Chemother. 2017, 61, 10–1128.
- Zhang, Q.; Xing, S.Z.; Sun, Q.; Pei, G.Q.; Cheng, S.; Liu, Y.N.; An, X.P.; Zhang, X.L.L.; Qu, Y.G.; Tong, Y.G. Characterization and complete genome sequence analysis of a novel virulent Siphoviridae phage against Staphylococcus aureus isolated from bovine mastitis in Xinjiang, China. Virus Genes 2017, 53, 464–476.
- Titze, I.; Krömker, V. Antimicrobial Activity of a Phage Mixture and a Lactic Acid Bacterium against Staphylococcus aureus from Bovine Mastitis. Vet. Sci. 2020, 7, 31.
- Titze, I.; Lehnherr, T.; Lehnherr, H.; Krömker, V. Efficacy of Bacteriophages Against Staphylococcus aureus Isolates from Bovine Mastitis. Pharmaceuticals 2020, 13, 35.
- Breyne, K.; Honaker, R.W.; Hobbs, Z.; Richter, M.; Żaczek, M.; Spangler, T.; Steenbrugge, J.; Lu, R.; Kinkhabwala, A.; Marchon, B. Efficacy and safety of a bovine-associated Staphylococcus aureus phage cocktail in a murine model of mastitis. Front. Microbiol. 2017, 8, 2348.
- Brouillette, E.; Millette, G.; Chamberland, S.; Roy, J.P.; Ster, C.; Kiros, T.; Hickey, S.; Hittle, L.; Woolston, J.; Malouin, F. Effective Treatment of Staphylococcus aureus Intramammary Infection in a Murine Model Using the Bacteriophage Cocktail StaphLyse™. Viruses 2023, 15, 887.
- Porter, J.; Anderson, J.; Carter, L.; Donjacour, E.; Paros, M. In vitro evaluation of a novel bacteriophage cocktail as a preventative for bovine coliform mastitis. J. Dairy Sci. 2016, 99, 2053–2062.
- Principi, N.; Silvestri, E.; Esposito, S. Advantages and Limitations of Bacteriophages for the Treatment of Bacterial Infections. Front. Pharmacol. 2019, 10, 513.
- Nale, J.Y.; Chutia, M.; Carr, P.; Hickenbotham, P.T.; Clokie, M.R.J. ‘Get in Early’; Biofilm and Wax Moth (Galleria mellonella) Models Reveal New Insights into the Therapeutic Potential of Clostridium difficile Bacteriophages. Front. Microbiol. 2016, 7, 1383.
- Malik, D.J.; Sokolov, I.J.; Vinner, G.K.; Mancuso, F.; Cinquerrui, S.; Vladisavljevic, G.T.; Clokie, M.R.J.; Garton, N.J.; Stapley, A.G.F.; Kirpichnikova, A. Formulation, stabilisation and encapsulation of bacteriophage for phage therapy. Adv. Colloid. Interface Sci. 2017, 249, 100–133.
- Geng, H.; Zou, W.; Zhang, M.; Xu, L.; Liu, F.; Li, X.; Wang, L.; Xu, Y. Evaluation of phage therapy in the treatment of Staphylococcus aureus-induced mastitis in mice. Folia Microbiol 2020, 65, 339–351.
- Guo, M.; Gao, Y.; Xue, Y.; Liu, Y.; Zeng, X.; Cheng, Y.; Ma, J.; Wang, H.; Sun, J.; Wang, Z.; et al. Bacteriophage Cocktails Protect Dairy Cows Against Mastitis Caused By Drug Resistant Escherichia coli Infection. Front. Cell Infect. Microbiol. 2021, 11, 690377.
- Hamza, A.; Perveen, S.; Abbas, Z.; Rehman, S.U. The Lytic SA Phage Demonstrate Bactericidal Activity against Mastitis Causing Staphylococcus aureus. Open Life Sci. 2016, 11, 39–45.
- Bissong, M.E.A.; Ateba, C.N. Genotypic and Phenotypic Evaluation of Biofilm Production and Antimicrobial Resistance in Staphylococcus aureus Isolated from Milk, North West Province, South Africa. Antibiotics 2020, 9, 156.
- Fox, L.K.; Zadoks, R.N.; Gaskins, C.T. Biofilm production by Staphylococcus aureus associated with intramammary infection. Vet. Microbiol. 2005, 107, 295–299.
- Song, J.; Ruan, H.; Chen, L.; Jin, Y.; Zheng, J.; Wu, R.; Sun, D. Potential of bacteriophages as disinfectants to control of Staphylococcus aureus biofilms. BMC Microbiol. 2021, 21, 57.
- Pedersen, R.R.; Krömker, V.; Bjarnsholt, T.; Dahl-Pedersen, K.; Buhl, R.; Jørgensen, E. Biofilm Research in Bovine Mastitis. Front. Vet. Sci. 2021, 8, 656810.
- Shi, Y.; Zhao, W.; Liu, G.; Ali, T.; Chen, P.; Liu, Y.; Kastelic, J.P.; Han, B.; Gao, J. Bacteriophages isolated from dairy farm mitigated Klebsiella pneumoniae-induced inflammation in bovine mammary epithelial cells cultured in vitro. BMC Vet. Res. 2021, 17, 37.
- Ngassam-Tchamba, C.; Duprez, J.-N.; Fergestad, M.; De Visscher, A.; L’Abee-Lund, T.; De Vliegher, S.; Wasteson, Y.; Touzain, F.; Blanchard, Y.; Lavigne, R. In vitro and in vivo assessment of phage therapy against Staphylococcus aureus causing bovine mastitis. J. Glob. Antimicrob. Resist. 2020, 22, 762–770.
- Liang, B.; Zhao, W.; Han, B.; Barkema, H.W.; Niu, Y.D.; Liu, Y.; Kastelic, J.P.; Gao, J. Biological and genomic characteristics of two bacteriophages isolated from sewage, using one multidrug-resistant and one non-multidrug-resistant strain of Klebsiella pneumoniae. Front. Microbiol. 2022, 13, 943279.
- Gill, J.J.; Pacan, J.C.; Carson, M.E.; Leslie, K.E.; Griffiths, M.W.; Sabour, P.M. Efficacy and pharmacokinetics of bacteriophage therapy in treatment of subclinical Staphylococcus aureus mastitis in lactating dairy cattle. Antimicrob. Agents Chemother. 2006, 50, 2912–2918.
- O’Flaherty, S.; Coffey, A.; Meaney, W.J.; Fitzgerald, G.F.; Ross, R.P. Inhibition of bacteriophage K proliferation on Staphylococcus aureus in raw bovine milk. Lett. Appl. Microbiol. 2005, 41, 274–279.
- Wipf, J.R.; Schwendener, S.; Perreten, V. The novel macrolide-Lincosamide-Streptogramin B resistance gene erm(44) is associated with a prophage in Staphylococcus xylosus. Antimicrob. Agents Chemother. 2014, 58, 6133–6138.
- Chen, Y.; Batra, H.; Dong, J.; Chen, C.; Rao, V.B.; Tao, P. Genetic Engineering of Bacteriophages Against Infectious Diseases. Front. Microbiol. 2019, 10, 954.
- Gibb, B.; Hyman, P.; Schneider, C.L. The Many Applications of Engineered Bacteriophages—An Overview. Pharmaceuticals 2021, 14, 634.
- Guo, D.; Chen, J.; Zhao, X.; Luo, Y.; Jin, M.; Fan, F.; Park, C.; Yang, X.; Sun, C.; Yan, J.; et al. Genetic and Chemical Engineering of Phages for Controlling Multidrug-Resistant Bacteria. Antibiotics 2021, 10, 202.
- Aarestrup, F.Μ.; Wegener, H.C.; Rosdahl, V.T.; Jensen, Ν.E. Staphylococcal and other Bacterial Species Associated with Intramammary Infections in Danish Dairy Herds. Acta Vet. Scand. 1995, 36, 475–487.
- Aarestrup, F.M.; Wegener, H.C.; Rosdahl, V.T. A comparative study of Staphylococcus aureus strains isolated from bovine subclinical mastitis during 1952–1956 and 1992. Acta Vet. Scand. 1995, 36, 237–243.
- Aarestrup, F.M.; Wegener, H.C.; Rosdahl, V.T. Evaluation of phenotypic and genotypic methods for epidemiological typing of Staphylococcus aureus isolates from bovine mastitis in Denmark. Vet. Microbiol. 1995, 45, 139–150.
