The intestinal barrier is a precisely regulated semi-permeable physiological structure that absorbs nutrients and protects the internal environment from infiltration of pathological molecules and microorganisms. Bile acids are small molecules synthesized from cholesterol in the liver, secreted into the duodenum, and transformed to secondary or tertiary bile acids by the gut microbiota. Bile acids interact with bile acid receptors (BARs) or gut microbiota, which plays a key role in maintaining the homeostasis of the intestinal barrier.
1. Introduction
The intestinal barrier is a highly complex and precisely controlled physiological structure. It interacts with the external environment as a physical, biochemical, and immunological barrier and regulates many critical homeostatic functions [
1]. In health, the intestinal barrier is semi-permeable and protects the internal environment from the potential infiltration of pathological molecules and microorganisms while allowing for the absorption of nutrients and water [
2]. However, under pathological situations, such as ischemia, trauma, stress, and infection, the integrity of the intestinal barrier is disrupted, leading to many local and systemic diseases.
Bile acids are hydroxylated sterols derived from cholesterol in the liver via either the classical (neutral) or alternative (acidic) pathway. Primary bile acids are synthesized in the liver, stored in the gallbladder, and then secreted into the intestine. In the gut, bile acids are transformed into secondary or tertiary bile acids by the microbiota [
3]. Traditionally, bile acids were thought to be involved in the absorption and metabolism of lipid and lipid soluble vitamins. Recent data suggest that bile acids act as hormones and engage bile acid receptors (BARs), including farnesoid X receptor (FXR), Takeda G protein-coupled receptor 5 (TGR5), sphingosine-1-phosphate receptor 2 (S1PR2), pregnane X receptor (PXR), vitamin D receptor (VDR), and constitutive androstane receptor (CAR), to play important roles in the metabolism, inflammation, immune homeostasis, tumorigenesis, aging, and other aspects of organism [
4,
5,
6,
7,
8,
9].
2. Bile Acids and Intestinal Barrier
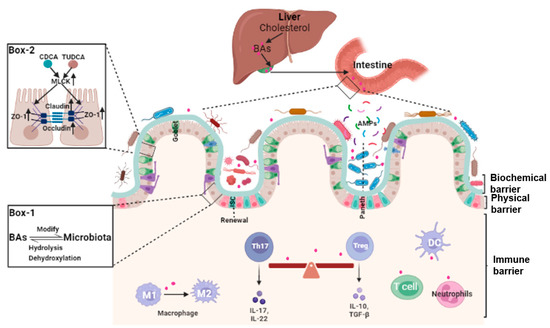
The integrity of the intestinal barrier requires constant renewal of the epithelial boundary, maintenance of tight junctions (physical barrier), mucus secretion and normal gut microbiota (biochemical barrier), and a finely regulated intestinal lamina propria immune system (immune barrier). Because of the complexity and heterogeneity of the intestinal barrier, specific mechanisms underlying the dysfunction of the intestinal barrier are still far from clear. That bile acids may play a pivotal role in many aspects of the maintenance of intestinal barrier integrity is a novel concept (Figure 1).
Figure 1. The roles of bile acids in the homeostasis of the intestinal barrier. Bile acids (BAs) are synthesized in the liver, stored in the gallbladder, and secreted into the intestine. In the gut, bile acids modify the growth of gut microbiota. Reciprocally, bile acids are dehydroxylated and/or de-conjugated by the gut microbiota to form secondary or tertiary bile acids (Box 1). Bile acids such as CDCA and TUDCA are involved in the maintenance of the integrity of the intestinal barrier through affecting the expression of tight junction proteins (Box 2). Bile acids are also involved in modifying the gut microbiota and intestinal mucosal lamina propria local immune system. In this location, they regulate macrophage polarization, inflammatory T helper 17 (Th17) cells and regulatory T cell (Treg) cells, and dendritic cells (DCs).
2.1. Bile Acids and Intestinal Epithelial Cells Tight Junctions
Tight junctions provide the main connection between intestinal epithelial cells and are formed by zonula occludens (ZOs), claudins (Cldns), and occludin (Ocln) proteins, which play an important role in maintaining the normal physiological function of epithelial cells [
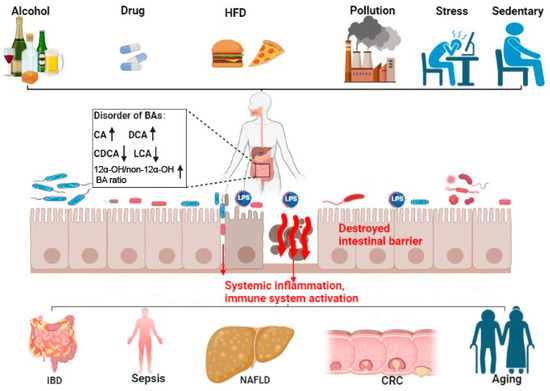
13]. The composition of bile acids is affected by diet, exercise, drugs, age, and other factors, and responds dynamically to local and whole-body ques (
Figure 2). Alteration of the bile acid profile changes the permeability of the intestinal mucosa and affects the barrier function through regulating the expression of tight junction proteins. For example, a high fat diet (HFD)-induced increase in deoxycholic acid (DCA) is a major environmental factor in the development of colorectal cancer (CRC). Apart from inducing chronic inflammation, reductions in zonula occludens 1 (ZO-1) and goblet and Paneth cells were observed in
Apcmin/+ mice after DCA treatment [
14].
Figure 2. Bile acid disorder and intestinal-barrier-dysfunction-related diseases. Bile acid (BA) metabolic disorder can be caused by alcohol intake, drugs, HFD, pollution, stress, and a sedentary lifestyle. Bile acid metabolic disorders can cause increased CA and DCA (shown by ↑), decreased CDCA and LCA (shown by ↓), and increased 12α-OH/non-12α-OH BAratio (↑). This imbalance in the BA profile can damage the intestinal barrier, increase the translocation of pathogenic microbiota and metabolites (shown by red arrows), and promote systemic inflammation and immune system activation. These then potentiate IBD, sepsis, NAFLD, CRC, and aging. HFD, high fat diet; LPS, lipopolysaccharides; CA, cholic acid; CDCA, chenodeoxycholic acid; DCA, deoxycholic acid; LCA, lithocholic acid; FXR, farnesoid X receptor; TGR5, Takeda G protein-coupled receptor 5; IBD, intestinal inflammatory diseases; NAFLD, non-alcoholic fatty liver disease; CRC, colorectal cancer.
2.2. Bile Acids and Gut Microbiota
Ursodeoxycholic acid (UDCA) supplementation attenuated inflammation and reduced intestinal permeability caused by multidrug-resistant extended-spectrum β-lactamase (ESBL)-producing
E. coli in colibacillus diarrhea. UDCA supplementation inhibited bacterial growth and invasion, alleviated commensal bacterial dysbiosis, and corrected colitis via the TGR5- nuclear factor-kappa B (NF-κB) pathway [
23]. Fibroblast growth factor FGF 15/19 (FGF15/19) is mainly expressed in the intestine under the control of the FXR. The activation of the FXR-FGF19 axis modulated intestinal flora and inhibited intestinal inflammation via restoring the normal bile acid pool [
24].
As gut microbiota constantly comes into contact with the external environment, the composition and function of the microbiota is susceptible to many factors, such as diet, medications, exercise, and emotions. In keeping with this, the consumption of an HFD modified the gut microbiome and bile acid pool and increased intestinal mucosal permeability [
25]. In mice, dietary fiber with insulin altered the composition of the microbiota and the levels of microbiota-derived metabolites, notably bile acids, that triggered type 2 inflammation at barrier surfaces [
10,
26].
2.3. Bile Acids, Intestinal Stem Cells (ISCs), and Epithelial Injury Repair
Balance between intestinal epithelial proliferation and cell death from damage, stress, and other pathological conditions maintain normal intestinal barrier function. Intestinal epithelial cells are self-renewed every 3–5 days, in part, from Lgr5+ intestinal stem cell (ISCs) as at counteract intestinal barrier damage and different stress stimuli [
30]. Lgr5+ ISCs replenish damaged epithelial cells and generate progenitors of goblet and Paneth cells. These cells secrete mucus and antimicrobial peptides to support the integrity of the intestinal mucus layer [
31].
Data demonstrated that bile acids metabolism plays a potential role in the self-renewal function of Lgr5+ ISCs. Based on pathway synthesis and chemical structures, bile acids are grouped into 12α-hydroxylated (OH) bile acids and non-12α-OH bile acids [
3]. What the two groups exert varies, and, at times, they have opposing effects on ISCs. For instance, the 12α-OH bile acid CA can inhibit the activity of peroxisome proliferator-activated receptor alpha (PPARα), impeding fatty acid oxidation (FAO), and the self-renewal of Lgr5+ ISCs [
32]. An HFD-driven increase in DCA decreased ISC proliferation and differentiate into goblet cells through pathologic endoplasmic reticulum stress [
33].
2.4. Bile Acids and Intestinal Local Immune Homeostasis
The intestinal lamina propria is colonized by a variety of innate and adaptive immune cells and gut-associated lymphoid tissue and is termed the intestinal immune barrier [
37,
38,
39]. Under normal conditions, this microecosystem is tightly and finely regulated. Environmental factors and the gut microbiota and their metabolites (microorganism-associated molecular patterns and pathogen-associated molecular patterns) are recognized by specific receptors (Toll-like receptors, TLRs) on immune cells, leading to intestinal immune homeostasis and self-tolerance [
39].
Bile acids modulate immune responses in the intestine through BARs, including TGR5, FXR, VDR, CAR, and retinoic-acid-receptor-related orphan nuclear receptor-γt (RORγt). Unique lymphocyte populations function cooperatively to maintain the intestinal immune system, especially the balance between pro-inflammatory T helper 17 (Th17) cells and anti-inflammatory Treg cells [
40]. Two derivatives of LCA, 3-oxoLCA and isoalloLCA, inhibited RORγt to suppress Th17 cell differentiation and increased the differentiation of Treg cells through the production of mitochondrial reactive oxygen species [
41]. The secondary bile acid, isoLCA, via VDR, modulated colonic FOXP3 + Treg cells expressing RORγt and ameliorated their susceptibility to inflammatory colitis [
42].
3. Bile Acid and Intestinal-Barrier-Dysfunction-Related Diseases
3.1. Inflammatory Bowel Diseases (IBDs)
Some bile acids altered the expression of tight junction proteins and the renewal of intestinal stem cells, leading to the intestinal barrier injury, increasing the incidence of IBD [
32,
50]. In contrast, some other bile acids may maintain intestinal immune barrier homeostasis by activating BARs such as FXR and TGR5. The DCA-mediated activation of FXR inhibited Paneth cell function and type I interferon signaling in mice with Crohn’s disease [
51]. Nuclear xenobiotic receptor CAR signaling altered the transcriptome of Teff cells that infiltrated the small intestine lamina propria (siLP) and suppressed Crohn’s disease-like small bowel inflammation [
80].
3.2. Gut Origin of Sepsis
The role of bile acids in intestinal barrier retention is well confirmed. However, there is also a close relationship between the metabolism of bile acids and gut-originating sepsis. Consistent with this view, the inflammatory mediators released during sepsis inhibited hepatobiliary transporter gene expression, resulting in hyperbilirubinemia and cholestasis [
90]. Serum bile acid concentrations were significantly higher in animals and humans with sepsis. Thus, bile acids may be a potential marker for early sepsis [
52,
53]. Of interest is the analysis of plasma from septic individuals found to be glycochenodeoxycholate- and phenylalanine-associated with survival of sepsis [
91].
3.3. Non-Alcoholic Fatty Liver Disease (NAFLD)
Bile acids may also affect the progression of NAFLD through adjusting the intestinal barrier [
105,
106,
107]. Long-term HFD intake can lead to gut dysbiosis and the aberrant metabolism of bile acids. Bidirectional crosstalk between gut microbiota and bile acids could impair the intestinal epithelial function, increasing the translocation of gut-derived endotoxins such as LPS to the blood and lymphatics to activate hepatic TLR-4/NF-κB signaling and promote NAFLD or NASH [
108,
109]. HFD-mediated changes in bile acids decreased FXR and TGR5 signaling and degraded the intestinal barrier [
110,
111].
3.4. Colorectal Cancer (CRC)
The dysregulation of bile acids-BAR pathways also plays a pivotal role in the progression of CRC. Decreased FXR-FGF15 signaling and overexpressed TGR5 were observed in azoxymethane (AOM)/dextran sodium sulfate (DSS)-induced CAC mice [
68]. In contrast, the activation of FXR protected the intestinal barrier, decreased inflammation, and restricted tumor growth [
126]. HFD and dysregulated Wnt signaling (APC mutation) altered bile acids profiles, increased the malignant transformation of Lgr5+ cancer stem cells, and promoted adenocarcinoma progression which was counteracted by the activation of FXR [
127].
3.5. Aging
Even though longevity is the people’s long-standing pursuit, to clarify the exact mechanism and pick out the ‘Mr. Key’ in this process is still the most important to success. Fortunately, many lines of evidence have clearly demonstrated that modifying the bile acids profile can delay aging effectively. A Mediterranean diet or calorie restriction altered the gut microbiota and bile acids, which was posited to improve health during aging [135,136,137,138]. In rodents, methionine restriction increased macroautophagy/autophagy and altered bile acid conjugation and levels to lengthen lifespan [139,140]. Modifying the bile acid profile with medications or fecal transplantation also relieved age-associated metabolic dysregulation in mice [141,142].
4. Conclusions
A well-functioning intestinal barrier maintains normal function of the digestive tract and positively impacts the entire individual. This is not surprising as the intestinal barrier is the largest interface between the individual and the environment. It is sensitive to changes in many external and internal factors such as diet, pollution, alcohol, drugs, stress, and life cycle [
143,
144,
145]. Bile acids are the only small molecules synthesized de novo and metabolized by the digestive system. Bile acids traverse the enterohepatic circulation 6–8 times per day and have a complex dynamic interaction with the gut microbiota [
3]. It is reasonable to ascribe a central role to bile acids in intestinal homeostasis.
Given the physiological significance of bile acids signaling, greater insight into the complex relationship between bile acids and the intestinal barrier could uncover safe therapies for intestinal, hepatobiliary, and age-related diseases. Encouraging is the considerable progress achieved in modifying bile acid signaling through the use of bile acids and their derivatives (e.g., UDCA and OCA), targeting BARs, and through the regulation of the gut microbiota (e.g., by fecal microbiota transplant). However, considering interindividual variability in disease status and tolerance to treatment, personalized medicines are still in need.
Bile acid metabolism is a multi-step physiological process, except for 12a-hydroxylated, many other modification processes, such as 7a-dehydroxylation, hydrolysis, and epimerization, may also play significant roles in the physiochemical and signaling properties of BAs. For example, some derivatives of LCA, formed by isomerization, can affect the function of the intestinal immune barrier through suppressing Th17 cell differentiation and increasing the differentiation of Treg [
40,
41]. Even the composition of BAs in mice differs from that of humans because the majority of CDCA in mice is typically converted to MCA [
135]. But, according to the most recent research, both have been reported to improve the function of gut barrier [
18,
146,
147].
In summary, fine-tuning the metabolism of bile acids is important in the homeostasis of the intestinal barrier. Disorders of bile acids and BAR signaling are involved in intestinal barrier dysfunction. Targeting bile acids and bile acid pathways may provide treatments to the related diseases arising from the deterioration of the intestine barrier. Still, further studies are warranted to elucidate the underlying mechanisms of action. Large, controlled, longitudinal clinical studies will assist in this.
This entry is adapted from the peer-reviewed paper 10.3390/cells12141888