Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Oncology
Metallic nanoparticles are promising nanomaterials in cancer therapy; however, functionalization of these nanoparticles with biomolecules has become relevant as their effect on cancer cells is considerably increased by photothermal and photodynamic therapies, drug nanocarriers, and specificity by antibodies, resulting in new therapies that are more specific against different types of cancer.
- cancer
- functionalized nanoparticles
- metallic nanoparticles
1. Introduction
Metallic NPs have a typical core/shell structure. The core is composed of a metal that determines the properties of the NPs, such as fluorescence, optical, magnetic, and electronic properties. The shell is composed of metals or organic polymers that protect the metallic core from chemical interactions with the environment and give the NPs the ability to be conjugated with some biomolecules [20]. These include: the low molecular weight ligands, peptides, proteins, polysaccharides, polyunsaturated and saturated fatty acids, deoxyribonucleic acid (DNA), plasmids, small interfering ribonucleic acid (siRNA), antibodies, tumor markers, and small molecules (Figure 1) [21]. Metallic NPs are often used as drug delivery systems or molecules that function as therapeutic agents through functionalization [22]. Recently, nanoparticle-based recombinant RNA and DNA delivery has been used in gene therapy, mainly because of its high transfection efficiency, low immunogenicity, biocompatibility, and most importantly, protection of genetic material from enzymatic degradation [23]. The entry of NPs into the cellular cytoplasm is very important for them to exert their effects. These nanostructures can be internalized by clathrin-mediated endocytosis, lipid raft/caveolae-mediated endocytosis, and micropinocytosis. The interaction that must take place between the NPs and the cell is very important. Some factors depend on this interaction, such as size, shape, surface charge, lipophilicity, type of nanocarrier, and finally the cell involved in the internalization [24]. The size of the NPs is an important factor in the internalization process because NPs with diameters between 10 and 100 nm are internalized via clathrin and via caveolae [25] to be incorporated into the intracellular traffic.
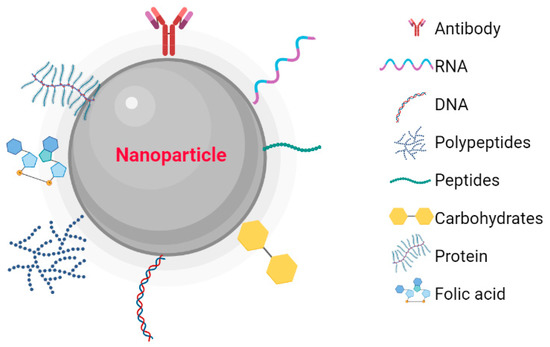
Figure 1. Nanoparticles functionalized with biomolecules: Antibodies, RNA, DNA, Polymers, Peptides, Carbohydrates, Proteins, and Folic Acid. Created with BioRender.com.
2. Metallic Nanoparticles
2.1. Palladium Nanoparticles
Pd is a noble metal and has particular characteristics, such as high thermal and chemical stability, catalytic activity, and an adjustable optical response, so it has high potential for radioactive image acquisition and photothermal therapy, suggesting a limited application in biomedicine [26]. However, some studies suggest that palladium nanoparticles (PdNPs) have therapeutic potential in cancer through photothermal and photodynamic therapies because this material absorbs in Near-Infrared spectroscopy (NIR), so strategies have been developed using NIR laser photoabsorbers to generate heat under NIR laser irradiation, resulting in highly specific cancer therapy. For example, Thapa et al. (2018) used PdNP-decorated graphene oxide (GO) (GO-PdNPs) to treat a solid prostate cancer tumor in a murine model, observing that intratumorally administered GO-PdNPs exerted a high photothermal effect, i.e., generation of reactive oxygen species (ROS). This is due to the high retention of GO-PdNPs in the solid tumor and the property of GO to absorb in the near-infrared region, which induces photoelectron interactions to generate heat, leading to photothermal ablation of the tumor [27].
2.2. Gold Nanoparticles
Gold has a high atomic number, which causes gold nanoparticles (AuNPs) to absorb more photons than soft tissue [33], have a wide range of applications in both the diagnosis and treatment of cancer due to their high bioavailability and biocompatibility, ease of synthesis and bioconjugation, and chemical and physical properties [34,35,36]. Particularly important features include localized surface plasmon resonance (LSPR), radioactivity, and high X-ray absorption coefficient. However, surface-enhanced fluorescence (SEF), photothermal transformation, photochemical transformation, and colorimetric reactions stand out in diagnosis [37]. In addition, its physical properties such as size and shape can affect absorption at the cellular level, leading to better results when incorporated into nanospheres, which have this ideal shape due to their high surface-to-volume ratios and low toxicity [33].
In addition, their chemical properties have the ability to form stable chemical bonds with groups containing sulfur and nitrogen (S and N, respectively) in their structure. Through these bonds, the surface of AuNPs can be functionalized with a variety of ligands, such as antibodies [38,39,40], proteins [41,42,43], peptides [44,45], and other biomolecules to obtain biologically active conjugates.
2.3. Silver Nanoparticles
The biological activity of silver nanoparticles (AgNPs) depends on factors including: surface chemistry, size, shape, morphology, composition, and reactivity in solution. These properties are crucial factors for their cytotoxic activity [63]. Another important feature is their ability to conjugate different types of ligands to their surface to increase their specificity and exert an effect [64]. Currently, the functionalization of AgNPs is aimed at developing targeted treatments to improve their effect on target cells. Functionalization includes antibodies [65,66], carbohydrates [67,68], polysaccharides [69], proteins [70,71], folic acid [72,73,74], and other molecules. AgNPs have shown high potential as anticancer agents due to their nanometric nature. These nanoparticles can actively or passively penetrate tumor tissue, where they can accumulate. Passive accumulation is due to the particular cytoarchitecture of tumor tissue, which includes an atypical endothelial layer and the absence of tissue-forming vessels and pores between 100 nm and 2 µm in diameter, resulting in the accumulation of nanometer-sized materials in cancer tissue. This phenomenon is referred to as the enhanced permeability and retention (EPR) effect [75]. On the other hand, active targeting has been proposed to increase the specificity of AgNPs against cancer. This specificity is based on a biological interaction between the ligands on the surface of the NPs and the target cell [76].
The cytotoxic effect of AgNPs is mainly due to the generation of ROS and oxidative stress, DNA damage, cell cycle arrest, and induction of tumor cell death by apoptosis [77,78,79]. The uptake of AgNPs by cells increases the synthesis and accumulation of ROS at the intracellular level, resulting in damage to various cellular components, degradation of DNA, lipid peroxidation, and carbonylation of proteins, causing oxidative stress-induced apoptosis and damaging the components of the cell [80,81]. Apoptosis is triggered by the release of Ag ions in the cytosol, which is promoted by the acidic lysosomal environment and increases the permeability of the mitochondrial membrane, leading to mitochondrial dysfunction and eventually cell apoptosis [82]. Several studies suggest that AgNPs are able to affect the expression of proteins that regulate the cell cycle. These proteins include cyclins B, E, and D, as well as up-regulation of p53 phosphorylation, leading to cell cycle arrest in the G2/M phase and increased production of ROS and the eventual induction of apoptosis [83].
The generation of anticancer therapeutic targets by functionalizing AgNPs has led to the development of specific agents with antitumor potential and lower toxicity to normal tissues. In one study, functionalization of AgNPs with monoclonal antibodies targeting EGFR (AgNPs-Anti-EGFR) was investigated in human nasopharyngeal carcinoma epithelial (CNE) cells and found that AgNPs-Anti-EGFR inhibited cell growth, induced apoptosis, increased sensitivity to radiation, and decreased expression of DNA damage/repair proteins such as Ku-70, Ku-80, and Rad51. This suggests that AgNPs-Anti-EGFR have a radiosensitizing effect as well as an antiproliferative effect on CNE cells [84]. Another study demonstrated the efficacy of affibody ZHER2:342-conjugated AgNPs (AgNPs-HER2) in cancer therapy by investigating these nanoparticles on HER2 overexpressing cancer cells and on a xenograft tumor model. Human ovarian adenocarcinoma SKOV3-1ip cells overexpress HER2 and Chinese hamster ovary (CHO) cells as negative control. These results showed that the binding of AgNPs-HER2 in SKOV3-1ip cells compared with CHO cells was 10.6-fold higher. The interaction between cancer cells with AgNPs-HER2 and irradiation LED matrix (465 nm; power of 95 mW/cm2) exerts a cytotoxic effect, which is mainly caused by the increased production of ROS. However, cytotoxicity assays do not reflect the effect in an in vivo model. When AgNPs-HER2 followed by light irradiation was evaluated on the solid tumor, 100% inhibition of tumor growth was demonstrated, and metastasis was also inhibited [85].
2.4. Platinum Nanoparticles
Pt is a noble metal with significant catalytic activity and has anticancer activity itself [94]. Platinum nanoparticles (PtNPs) have recently gained attention due to their wide range of applications such as biosensor, electro-analytical and analytical, and catalysis, standing out in biological applications [95]. These materials have catalytic [96] and enzymatic activity and are inherently biocompatible, suggesting that they can be used as anticancer agents [97]. PtNPs have been shown to possess some intrinsic cytotoxic activity that slows tumor growth. However, the specificity is enhanced by functionalization of the nanoparticles, which increases their anticancer cytotoxic capacity [98].
Recently, the cytotoxic effect of a system of octopod-shaped PtNPs functionalized with doxorubicin (PtNPs-DOX) was demonstrated by the release of the chemotherapeutic agent in cancer cells (MCF-7 and MDA-MB-231). The effect is mediated by activation of the tumor suppressor gene (PTEN), which restricts the PI3K/AKT signaling pathway, leading to mitochondrial dysfunction and activation of caspases three and nine, ultimately resulting in cell apoptosis [99].
Functionalization of PtNPs with biopolymers is a therapy directed against tumors. It is known that some tumor cells overexpress receptors for hyaluronic acid (HA) (CD44) [101]. On this basis, an in vitro study showed that PtNPs encapsulated in hyaluronic acid (HA-PtNPs) accumulate in tumor cells, overexpressing CD44 (MDA-MB-231 cells) when compared with nonfunctionalized PtNPs and in cells not expressing CD44 (NIH3T3 cells). However, this accumulation has no cytotoxic effects. On the other hand, the effect of HA-PtNPs in combination with directed photothermal therapy (PTT) was investigated in an in vitro assay. This showed a cytotoxic effect on MDA-MB-231 cells, suggesting that PTT in combination with HA-PtNPs is more efficient in cells overexpressing CD44. Moreover, an in vivo study in mice with MDA-MB-231 breast tumors showed that intravenous administration of HA-PtNPs can be detected in the tumor and that after NIR irradiation, the temperature in the tumor is increased, leading to a decrease in tumor size [102].
3. Conclusions
NPs are proposed as new specific treatment alternatives for some cancers, as some studies have shown a cytotoxic effect on cancer cells and solid tumors, through cell cycle arrest, oxidative stress, ROS production, DNA damage, or death by apoptosis. Functionalization of NPs with other biomolecules may be a useful strategy for targeted, specific, effective, and, most importantly, noninvasive cancer therapy in healthy tissues.
This entry is adapted from the peer-reviewed paper 10.3390/pharmaceutics15071932
This entry is offline, you can click here to edit this entry!
