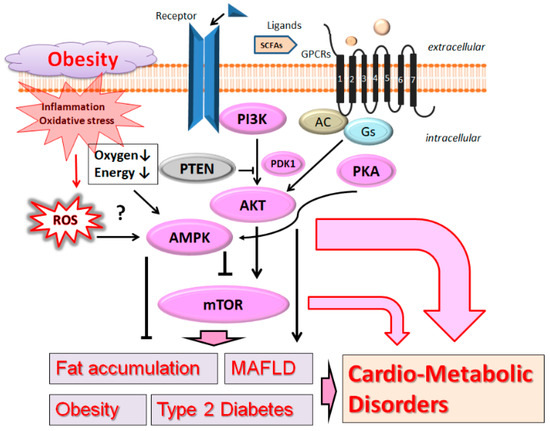
Dyslipidemia has been established as an important factor that is connected with the pathogenesis of several diseases including atherosclerosis, hypertension, cardiovascular disease, obesity, and diabetes mellitus. Therefore, cardio-metabolic disorders are a set of pathophysiological conditions resulting from a disturbance in hepatic lipid metabolism, which may occupy a position at the crossroads of cardiovascular disease and metabolic disorder [
1]. The risk factors of cardio-metabolic disorders may also enhance the morbidity rate for several diseases such as obesity, metabolic syndrome, hypertension, type 2 diabetes (T2D), ischemic heart disease, and stroke [
2]. In particular, obesity is now prevalent worldwide following an increase in comorbidities with various metabolic dysfunction including the T2D, metabolic-dysfunction-associated fatty liver disease (MAFLD), and cardio-metabolic disorders [
3] (
Figure 1). Here, the term “MAFLD” is used instead of the previous term NAFLD (non-alcoholic fatty liver disease), because MAFLD has been suggested as a more appropriate overarching term [
4]. Since cardio-metabolic disorders must have existed before they became well-known worldwide, it is crucial for the prevention of diseases to be informed of some altered tactics, as well as treatment arrangements, to regulate the prevalence [
5]. Among them, certain types of diet as good therapy for cardio-metabolic disorders could provide beneficial effects for the risk factors. In fact, there is an increasing interest for many researchers in the association between dietary glycemic index and the risk factors of cardio-metabolic disorders. MAFLD is one of the most common liver diseases in the world, in which other cardio-metabolic disorders in patients with MAFLD are significantly prevalent [
6]. Interestingly, the associations of metabolic disorders with MAFLD have been greater in non-obese than in obese patients with diabetes mellitus [
7]. A chronic pro-inflammatory condition in obesity patients could trigger and/or promote cardio-metabolic disorders [
8]. Chronic inflammation is also a risk factor for several conspicuous health concerns related to cardio-metabolic disorders [
9]. In addition, lifestyle and/or epigenetic factors may be also involved in the development of MAFLD and cardio-metabolic disorders [
10].
2. PI3K/AKT and AMPK Pathway Involved in Obesity, MAFLD, and Diabetes
Phosphoinositide 3-kinase (PI3K) and AKT, a serine/threonine protein kinase also known as protein kinase B, are key components with the mechanistic target of rapamycin (mTOR) for signaling network [
17], possibly related to the pathogenesis of cardio-metabolic disorders. The mTOR is also a serine/threonine kinase located downstream of the PI3K/AKT signaling pathway [
18]. (
Figure 1) Aberrant activation of the PI3K/AKT/mTOR network may contribute to pathological conditions including T2D, MAFLD, and cancers. Therefore, modulating the components in the PI3K/AKT/mTOR pathway has been proposed as a potential therapeutic option for preventing and/or treating diverse types of host disorders, whose chronic complications are substantial burdens in social communities [
19].
Glucose uptake through GLUT4 might be stimulated by anthocyanin via the upregulation of the PI3K/AKT and adenosine monophosphate (AMP)-activated protein kinase (AMPK) signaling pathways [
31]. AMPK is a widely existing protein kinase activated by AMP, which is a central regulator of cellular energy balance. It is well-known that AMPK plays an important role in fatty acid metabolism through the fatty acid biosynthetic pathway. In general, AMPK is located in liver and/or in muscle fibers, where AMPK might work as an indicator or a good marker for cellular energy balance. AMPK is also a metabolic sensor molecule regulating cellular energy metabolism, which might be also involved in anti-lipid metabolism [
32]. As a consequence, inhibition of AMPK may result in decreased fatty acid oxidation and increased fat accumulation, and vice versa [
33]. Suppressing the AMPK, therefore, could decrease the liver fatty acid oxidation in patients of obesity. In general, PTEN/PI3K and AMPK signaling pathways are modulated by classical antidiabetic drugs [
34]. AMPK activators, metformin, have been shown to prevent the development of hepatic steatosis [
35]. Consequently, AMPK is closely associated with the content of liver lipid and/or insulin resistance [
36]. An experimental study shows that protein expression levels of AMPK in high-fat-diet-induced MAFLD is considerably lower than that of the normal control group [
37], which may suggest that AMPK is actually involved in the pathogenesis of MAFLD.
3. Probiotics for the Modulation of PI3K/AKT and AMPK Pathway
Probiotics, as naturally occurring bacteria that are detected in human and/or animal intestine, revealed their capability against the development of various diseases via the alteration of intracellular signaling pathways within target cells. Importantly, the PI3K/AKT signaling pathway could be successfully modulated by probiotics [
38], which might offer important therapeutic options by targeting cellular apoptosis, survival, and/or protection. For example, modulation of the PI3K/AKT pathway has a considerable hepatoprotective mechanism, which could be achieved after
Akkermansia muciniphila (
A. muciniphila) administration [
39].
Lactiplantibacillus plantarum (
L. plantarum) treatment could also enhance the expression of PI3K/AKT in the liver, which could prevent high-fat-diet-induced glucose tolerance and/or hyperglycemia to improve type 2 diabetes mellitus [
40,
41]. Altered gut microbiota components might influence liver glycogen and/or muscle glycogen by elevating the mRNA expression of PI3K and/or AKT in the liver through the modulation of favorable bacteria such as
Lactobacillus sp. [
42].
Lactobacillus rhamnosus (
L. rhamnosus) could prevent cytokine-induced cellular apoptosis in gut epithelial cells by up-regulating the PI3K/AKT signaling pathway [
43]. Probiotic supplementation is a potential way to protect against high-fat-diet-induced and radiation-induced liver damage, possibly via the alteration of the PI3K/AKT signaling pathway [
44].
Bifidobacterium animalis subsp.
lactis (
B. lactis) strain could retard an apoptosis of the colonic epithelial cells by up-regulating the PI3K/AKT signaling pathway [
45]. Interestingly,
B. lactis combined with
L. plantarum could regulate the growth of malignant glioma by suppressing the PI3K/AKT pathway in mice [
46].
As mentioned above, AMPK is a significant molecule involved in regulating biological energy metabolism [
47] and/or in the regulation of aging, which could activate downstream molecules such as sirtuin1 (SIRT1) to inhibit inflammation and/or oxidative stresses [
48].
L. plantarum could activate AMPK for reducing the production of ROS and inflammation, which might contribute to inhibiting atherosclerosis [
49]. On the other hand, a gut-microbiota-dependent metabolite could bring various cardiovascular diseases [
49]. It is well-known that alteration of immune cells balance might be related to the inflammatory response through the AMPK and/or PI3K/AKT signaling pathway (
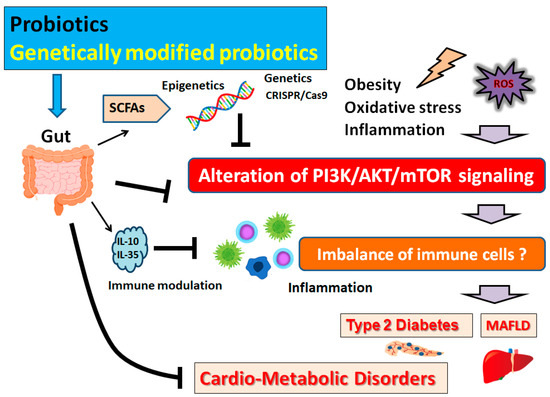
Figure 2).
Figure 2. Schematic representation of the inhibition of the pathogenesis of cardio-metabolic disorders. Some types of probiotics and/or genetically modified probiotics could contribute to the alteration of gut microbial community for playing valuable roles in inhibiting the PI3K/AKT/mTOR signaling pathway in part via the epigenetics regulation with SCFAs. Examples of certain beneficial microbial species with several effects on anti-cancer immune responses are shown on the left side. Arrowhead indicates stimulation whereas hammerhead shows inhibition. Note that several important activities such as cytokine induction and/or inflammatory reactions have been omitted for clarity. Abbreviation: MAFLD, metabolic-associated fatty liver disease; mTOR, mammalian/mechanistic target of rapamycin; PI3K, phosphoinositide-3 kinase; ROS, reactive oxygen species; SCFAs, short-chain fatty acids.
4. Possible Mechanism behind the Beneficial Effects of Probiotics on Obesity and Diabetes
Living microorganisms may have a valuable effect, with probiotics being beneficial for gut microbiota in human health [
56]. Therefore, gut microbiota and/or probiotics could be an important mediator for a communication between diet and host health [
57]. Microbial metabolites can influence host gene expression through the epigenetic mechanisms [
58]. In addition, the mechanisms affecting the expression of PI3K/AKT and AMPK signaling molecules could be associated with the alteration of cardio-metabolic parameters [
59]. Microbial metabolites such as SCFAs could influence the epigenetic programming by inhibiting histone deacetylase (HDAC) enzymatic activity [
60], which promotes de-condensation and relaxation of chromatin and increases the chromatin accessibility to various transcription factors [
61].
Faecalibacterium prausnitzii is one of the most abundant anaerobic bacteria in the gut of healthy individuals that can produce SCFAs such as butyrate. A strain of
Lactobacillus paracasei was initially isolated from the feces of an elderly Italian person, which could prevent and/or improve the pathology of type 2 diabetes mellitus by reduction in inflammation via the alteration of the PI3K/AKT pathway and the production of gut-microbiota-derived metabolites such as SCFAs [
62] (
Figure 2). Acetate, butyrate, and vitamins all play important roles in epigenetic regulation, which are mainly byproducts of the gut microbiota. After dietary intervention with
L. rhamnosus, the concentration of acetic acid, propionic acid, and butyric acid might be significantly raised [
63]. Therefore, it might be a potentially valuable choice to employ
L. rhamnosus for acquiring epigenetical effects in probiotics. Obesity is a complex pathology with a multifactorial pathogenesis linked with lifestyle and epigenetic factors [
64].
L. rhamnosus supplementation use as a probiotic could contribute to a significant decrease in plasma triglycerides, low density lipoprotein (LDL)-cholesterol, insulin, and homeostatic model assessment for insulin resistance (HOMA-IR) [
65]. In addition,
L. rhamnosus could bring improvement in the profile of total cholesterol, LDL, high density lipoprotein (HDL), triglycerides, and weight gain [
66].
L. rhamnosus could activate the AMPK pathway, and reduce the gene expression of peroxisome proliferator-activated receptor (PPAR) [
63]. In addition,
L. rhamnosus could down-regulate the expression of genes related to adipogenesis and/or lipogenesis in high-fat-diet-fed obese mice [
67].
One of the SCFAs, butyrate, a major end product of the bacterial fermentation of indigestible carbohydrates, might have positive effects on body weight control and insulin sensitivity [
68]. Sodium butyrate could attenuate the obesity-induced insulin resistance, fatty liver, and intestinal dysfunction [
69]. Butyrate has corrected hyperinsulinemia, lowered plasma leptin levels, and attenuated adipose tissue inflammation without affecting gut microbiota composition [
70]. Sodium butyrate is a short-chain fatty acid with HDAC inhibition activity, which could epigenetically promote beta-cell development, proliferation, and function, as well as improve glucose homeostasis [
71]. Many commensal gut bacteria such as
Lactobacillus and
Bifidobacterium species are important in folic acid production [
72], which might be involved in methylation changes as the promoters for regulating transcription activity [
73]. Understanding the precise and specific epigenetic changes in host genes associated with a cardio-metabolic disorder by certain microbiota could be important in the development of novel therapies against the cardio-metabolic disorder.