Obstructive sleep-disordered breathing (SDB) is a spectrum of different clinical conditions distinguished by upper-airway dysfunction during sleep with snoring and/or increased respiratory effort due to increased upper airway resistance and pharyngeal collapsibility. Diagnostic methods for SDB in children involve a combination of clinical assessment, medical history evaluation, questionnaires, and objective measurements. Polysomnography (PSG) is the diagnostic gold standard. It records activity of brain and tibial and submental muscles, heart rhythm, eye movements, oximetry, oronasal airflow, abdominal and chest movements, body position. Despite its accuracy, it is a time-consuming and expensive tool.
- sleep-disordered breathing
- overnight polysomnography
- diagnosis
- children
- respiratory events
1. Introduction
2. Polysomnography
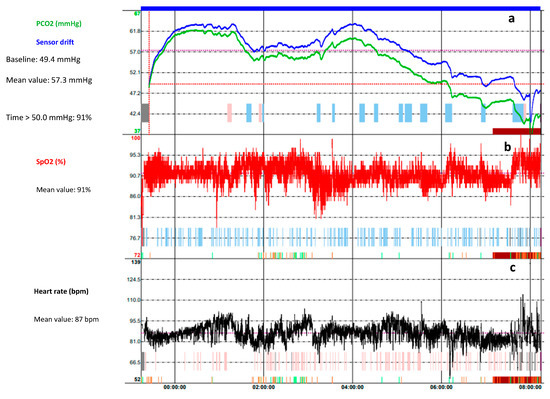
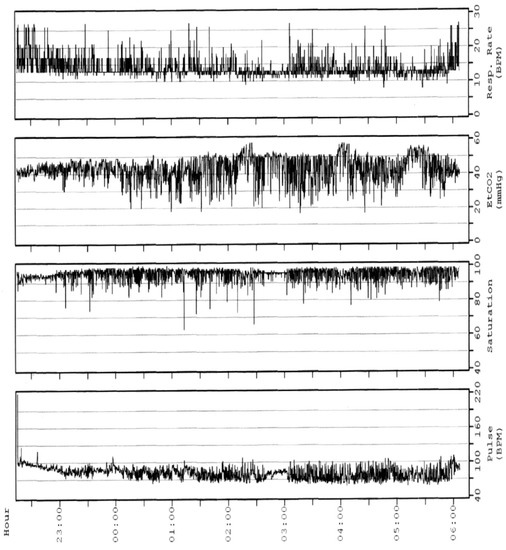
This entry is adapted from the peer-reviewed paper 10.3390/children10081331
References
- Kaditis, A.G.; Alonso Alvarez, M.L.; Boudewyns, A.; Alexopoulos, E.I.; Ersu, R.; Joosten, K.; Larramona, H.; Miano, S.; Narang, I.; Trang, H.; et al. Obstructive Sleep Disordered Breathing in 2- to 18-Year-Old Children: Diagnosis and Management. Eur. Respir. J. 2016, 47, 69–94.
- Amin, R.; Somers, V.K.; McConnell, K.; Willging, P.; Myer, C.; Sherman, M.; McPhail, G.; Morgenthal, A.; Fenchel, M.; Bean, J.; et al. Activity-Adjusted 24-Hour Ambulatory Blood Pressure and Cardiac Remodeling in Children with Sleep Disordered Breathing. Hypertension 2008, 51, 84–91.
- Lee, P.-C.; Hwang, B.; Soong, W.-J.; Meng, C.C.L. The Specific Characteristics in Children with Obstructive Sleep Apnea and Cor Pulmonale. Sci. World J. 2012, 2012, 757283.
- Bourke, R.; Anderson, V.; Yang, J.S.C.; Jackman, A.R.; Killedar, A.; Nixon, G.M.; Davey, M.J.; Walker, A.M.; Trinder, J.; Horne, R.S.C. Cognitive and Academic Functions Are Impaired in Children with All Severities of Sleep-Disordered Breathing. Sleep Med. 2011, 12, 489–496.
- Lumeng, J.C.; Chervin, R.D. Epidemiology of Pediatric Obstructive Sleep Apnea. Proc. Am. Thorac. Soc. 2008, 5, 242–252.
- Fund, N.; Green, A.; Chodick, G.; Orin, M.; Koren, G.; Shalev, V.; Dagan, Y. The Epidemiology of Sleep Disorders in Israel: Results from a Population-Wide Study. Sleep Med. 2020, 67, 120–127.
- Kara, C.O.; Ergin, H.; Koçak, G.; Kiliç, I.; Yurdakul, M. Prevalence of Tonsillar Hypertrophy and Associated Oropharyngeal Symptoms in Primary School Children in Denizli, Turkey. Int. J. Pediatr. Otorhinolaryngol. 2002, 66, 175–179.
- Castronovo, V.; Zucconi, M.; Nosetti, L.; Marazzini, C.; Hensley, M.; Veglia, F.; Nespoli, L.; Ferini-Strambi, L. Prevalence of Habitual Snoring and Sleep-Disordered Breathing in Preschool-Aged Children in an Italian Community. J. Pediatr. 2003, 142, 377–382.
- Borrelli, M.; Corcione, A.; Rongo, R.; Cantone, E.; Scala, I.; Bruzzese, D.; Martina, S.; Strisciuglio, P.; Michelotti, A.; Santamaria, F. Obstructive Sleep Apnoea in Children with Down Syndrome: A Multidisciplinary Approach. J. Pers. Med. 2022, 13, 71.
- Canora, A.; Franzese, A.; Mozzillo, E.; Fattorusso, V.; Bocchino, M.; Sanduzzi, A. Severe Obstructive Sleep Disorders in Prader-Willi Syndrome Patients in Southern Italy. Eur. J. Pediatr. 2018, 177, 1367–1370.
- Koyuncu, E.; Türkkani, M.H.; Sarikaya, F.G.; Özgirgin, N. Sleep Disordered Breathing in Children with Cerebral Palsy. Sleep Med. 2017, 30, 146–150.
- Ramezani, R.J.; Stacpoole, P.W. Sleep Disorders Associated with Primary Mitochondrial Diseases. J. Clin. Sleep Med. 2014, 10, 1233–1239.
- Verhulst, S.L.; Van Gaal, L.; De Backer, W.; Desager, K. The Prevalence, Anatomical Correlates and Treatment of Sleep-Disordered Breathing in Obese Children and Adolescents. Sleep Med. Rev. 2008, 12, 339–346.
- Marcus, C.L.; Brooks, L.J.; Draper, K.A.; Gozal, D.; Halbower, A.C.; Jones, J.; Schechter, M.S.; Sheldon, S.H.; Spruyt, K.; Ward, S.D.; et al. Diagnosis and Management of Childhood Obstructive Sleep Apnea Syndrome. Pediatrics 2012, 130, 576–584.
- Xu, Z.; Wu, Y.; Tai, J.; Feng, G.; Ge, W.; Zheng, L.; Zhou, Z.; Ni, X. Risk Factors of Obstructive Sleep Apnea Syndrome in Children. J. Otolaryngol. Head Neck Surg. 2020, 49, 11.
- Grigg-Damberger, M.; Gozal, D.; Marcus, C.L.; Quan, S.F.; Rosen, C.L.; Chervin, R.D.; Wise, M.; Picchietti, D.L.; Sheldon, S.H.; Iber, C. The Visual Scoring of Sleep and Arousal in Infants and Children. J. Clin. Sleep Med. 2007, 3, 201–240.
- Scholle, S.; Zwacka, G. Arousals and Obstructive Sleep Apnea Syndrome in Children. Clin. Neurophysiol. 2001, 112, 984–991.
- Berry, R.B.; Budhiraja, R.; Gottlieb, D.J.; Gozal, D.; Iber, C.; Kapur, V.K.; Marcus, C.L.; Mehra, R.; Parthasarathy, S.; Quan, S.F.; et al. Rules for Scoring Respiratory Events in Sleep: Update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events. Deliberations of the Sleep Apnea Definitions Task Force of the American Academy of Sleep Medicine. J. Clin. Sleep Med. 2012, 8, 597–619.
- Guilleminault, C.; Poyares, D.; Palombini, L.; Koester, U.; Pelin, Z.; Black, J. Variability of Respiratory Effort in Relation to Sleep Stages in Normal Controls and Upper Airway Resistance Syndrome Patients. Sleep Med. 2001, 2, 397–405.
- Amaddeo, A.; Fauroux, B. Oxygen and Carbon Dioxide Monitoring during Sleep. Paediatr. Respir. Rev. 2016, 20, 42–44.
