Microalgal biomass is currently considered as a sustainable and renewable feedstock for biofuel production (biohydrogen, biomethane, biodiesel) characterized by lower emissions of hazardous air pollutants than fossil fuels. Photobioreactors for microalgae growth can be exploited using many industrial and domestic wastes. It allows locating the commercial microalgal systems in areas that cannot be employed for agricultural purposes, i.e., near heating or wastewater treatment plants and other industrial facilities producing carbon dioxide and organic and nutrient compounds.
- microalgal biomass
- microalgae cultivation
- biofuels
- advantages
- limitations
1. Introduction
Microalgae are single-cell organisms that convert solar radiation energy into chemical energy via photosynthesis [1]. Controlled production of microalgal biomass is a fast-growing technology, as microalgae can be used to produce a wide range of commercially valuable cellular metabolites, including high-quality proteins, lipids, carbohydrates, dyes, and vitamins for the food/feed industry and the broad cosmetic industry.
The fact that microalgae represent an alternative and competitive source of biomass is due to their advantage over typical terrestrial and energy plants [2]. Algae possess very high photosynthetic efficiency [3], can relatively fast build biomass [4], are resistant to various contaminants [5], and can be grown on land that is unsuitable for other purposes [6]. Microalgae production systems can also be used in environment-protecting technologies [7], including sewage and leachate treatment [8], neutralization of waste and sludge [9], carbon dioxide biosequestration, biogas upgrading, and flue gas treatment [10]. This makes it possible to select and adapt specific strains for individual applications, including energy carrier production, environmental protection, and environmental engineering technologies [11]. Given these considerations, algae may provide a viable alternative to traditional energy crops [12].
2. Microalgal Biomass as a Source of Biofuels
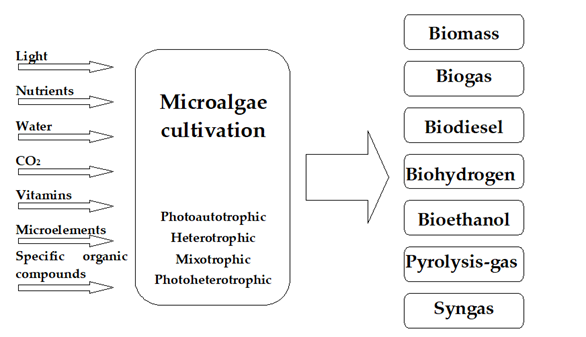
Microalgae can serve as a potential source of many different types of biofuels (Figure 2). Examples include anaerobic digestion of biomass into biogas, production of biodiesel from lipids stored in algae cells and hydrogen from photobiological conversion, and lastly, gasification, pyrolysis, or direct combustion of the harvested algal biomass [13][14].
Figure 2. Available mechanisms for producing biofuel with microalgae.
The simplest way to use microalgae for fuel purposes involves the combustion or co-combustion of their pre-dried biomass [15]. However, this solution is rarely practiced, most often in cases where the biomass of microalgae cannot be used to produce more advanced biofuels [16]. Biogas and biomethane are produced during controlled, anaerobic degradation of microalgal biomass by fermentation bacteria [17]. Methane fermentation is a cascade of successive biochemical transformations, including hydrolysis, acidogenesis, and methanogenesis, which are carried out by specialized consortia of microorganisms [18]. In turn, biodiesel is produced via the transesterification of bio-oil extracted from microalgal biomass. This process involves the reaction of triglyceride molecules, bio-oil components, with low-molecular-weight alcohols in the presence of catalysts [19]. Hydrogen production by microalgae is based on direct biophotolysis, which involves the photosynthetic production of hydrogen from water, which uses the energy of light to break down the water molecule into hydrogen and oxygen. The process is mediated by hydrogenase—a metal enzyme that catalyzes the reversible oxidation of H2 and releases gaseous hydrogen by reducing protons [20]. The basic technology for bioethanol production from microalgae entails a biochemical process in which bacteria hydrolyze the biomass and then yeast convert the sugars present in the biomass into alcohol, which is then distilled and dehydrated [21]. In turn, syngas and pyrolytic gas are produced via the endothermal conversion of biomass into gas, which mainly consists of hydrogen, carbon monoxide, carbon dioxide, methane, and low-molecular-weight hydrocarbons [22]. The contribution of individual products, including their qualitative composition, depends mainly on the process conditions, such as temperature, reaction time, pressure, and biomass characteristics [23].
Many researchers have argued that methane fermentation is the most promising and effective method for producing energy from algae. Sialve et al. (2009) found that, given suitable operating conditions, methane fermentation as a primary method of algal biomass processing is more economical than systems that incorporate lipid extraction and anaerobic processing of post-extraction residues [24]. Other findings suggest that the balance of methane fermentation unit operations is the most effective in terms of both the economy of the process and the pollution levels [25]. Studies have indicated that methane fermentation may be the most practical means of converting algal biomass into energy. However, Börjesson and Berglund (2006) noted that energy inputs and environmental impact varied greatly between the different methane fermentation technologies [26]. As such, an environmental life-cycle assessment (LCA) is necessary for a complete and objective evaluation of each process [27].
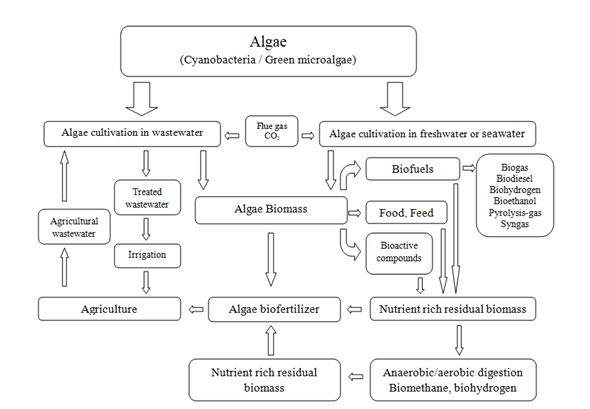
To meet the current challenges related to the circular bioeconomy, it is necessary to change the approach to biorefinery processes [28]. Technological, economic, and environmental efficiency improvements can be achieved by simultaneously producing many high-value products other than biofuels [29][30]. Research and development works must, therefore, be focused on finding new, more complex, and integrated production processes. Although various strategies have been proposed for converting algal biomass into fuel and fine chemicals, none have been proven to be economically viable and energy balanced [186]. Therefore, other, valuable biological products should also be searched for. In this context, the concept of microalgae biorefineries emerged with the concept of recovering multiple products from one operating process. Considering the biorefinery complexity index (BCI) as an indicator of technical and economic risk, one of the most promising seems to be the biorefinery platform based on microalgal biomass conversion into fuels, food, dietary and feed supplements, fertilizers, and pharmaceuticals [31]. A schematic diagram of a comprehensive biorefinery approach to the processing of microalgal biomass is presented below (Figure 3).
Figure 3. A schematic diagram of a comprehensive biorefinery approach to microalgal biomass processing.
3. Systems of Microalgae Species Cultivation for Biofuel
The growth rate of microalgae and their composition is influenced by the growth conditions and the species employed [32][33][34]. Many classification schemes categorize methods and technologies used to cultivate algae for biofuel [35][36]. Due to the specific nature of microalgae, the most important scheme divides the systems on the basis of the nutrient source and the type of biochemical processes used to grow the algal biomass rapidly. With this criterion in mind, cultures can be divided into four main types: photoautotrophic, heterotrophic, mixotrophic, and photoheterotrophic [37].
4. Strengths and Weaknesses of Different Technologies for Producing and Utilizing Microalgal Biomass
Microalgae-based technologies of sewage treatment, pollutant degradation, and biofuel production were described in detail in scientific papers, patent claims, and performance data from existing installations [38][39][40]. Microalgal biomass has been demonstrated to be one of the most efficient and environmentally friendly alternative energy sources, as it is a promising and sustainable source of bio-oil, methane, and biohydrogen, i.e., fuels that can help reduce atmospheric greenhouse gas emissions [41][42]. Microalgae represent an alternative to terrestrial vascular plant species commonly used as a biofuel feedstock, such as rapeseed, soybean, and oil palm [43]. Literature data indicate that the annual hectare yield of bio-oil from microalgal cultures can exceed 19 m3. By comparison, the corresponding values are 6.1 m3 for oil palm, 4.3 m3 for sugar cane, 2.4 m3 for corn, and 0.5 m3 for soybean [44].
The undeniable strength of the microalgae-based technologies is their well-established high photosynthetic efficiency. The efficiency of the solar-to-chemical energy conversion via algal photosynthesis varies from 4% to 10%, whereas the range for higher plants is 0.5–2.2% [45]. This directly translates to a fast growth rate of microalgae and a high per unit dry matter yield, significantly higher than that of terrestrial plants [43]. Those observations were corroborated by Tredici et al. (2015), who tested the strain Tetraselmis suecica in a proprietary photobioreactor design named “Green Wall Panel-II” The research was conducted in Italy (Tuscany), with the final productivity of the culture reaching 36 tons of dry microalgal biomass·ha−1·year−1. By contrast, soybean grain yields are only 2.6 tons·ha−1·year−1 [46].
Some strains of microalgae can double their mass in just a few hours. This property was described by Maxwell et al. (1994), who tested the growth rate of Chlorella vulgaris. The generation time observed for the species was 8.6 h at 27 °C, although cell division extended to 48.5 h at 5 °C [47]. Raslavicius et al. (2014) and Chen et al. (2015) showed that the annual microalgal biomass yields per hectare can range from 4 tons of dry matter to as high as 100 tons of dry matter [38][48]. According to other works, microalgae can double in volume or mass within a few hours, given the right conditions [41][43][45]. The resultant microalgal biomass yields can reach 500 kg·day−1 in a 1000 m2 open pond production system [49].
One indisputable advantage of the microalgae-based solutions is that waste substrates of various properties and characteristics can be used to support rapid biomass growth [50]. Such technologies are most often used for tertiary treatment of urban or industrial waste in maturation or facultative ponds [51]. Such organisms release 1.50–1.92 kg O2·kg−1 of the produced biomass through photosynthesis, with the oxygenation rate reached during degradation of organic pollutants ranging from 0.48 to 1.85 kg O2·m−3·day−1 [52][53]. Microalgae absorb a significant portion of the biogenic substances contained in wastewater, as they require large quantities of nitrogen and phosphorus for internal protein synthesis. As such, protein content in the algae dry matter ranges from 20% to 60%, depending on the species. The absorbed biogenic compounds are also used to synthesize nucleic acids and phospholipids [54].
Currently, microalgae-based wastewater treatment processes are often integrated into systems designed to grow algal biomass for biofuel and energy production [55]. Such solutions can be used to remove chemical and biological contaminants from wastewater, while concurrently growing biomass for biofuel production, thus proving to be more viable from the economic and technological standpoint [56]. The use of wastewater as a growth medium directly reduces the costs of supplying water and nutrients necessary for the algae to grow at an efficient rate [57]. Research so far has shown that high CO2 levels in wastewater promote microalgal growth, thus directly stimulating faster degradation of pollutants [58]. In systems where algae are grown in saltwater, the introduction of wastewater also serves to balance the molecular ratio of carbon, nitrogen, and phosphorus (C:N:P = 106:16:1), known as the Redfield ratio [59].
In light of the widely discussed effects of greenhouse gas emissions, integrated systems capable of reducing gas pollutant levels in the air, while simultaneously harvesting biomass and recovering energy, have attracted much interest [56]. One of the most promising and prospective avenues of evolving such systems lies in using microalgal biomass to remove pollutants from waste gases, mainly CO2, NO2, and SO2 [60][61]. Research to date has shown that intensive microalgae cultivation requires a supply of 1.83 kg CO2 per 1.0 kg of the grown dry matter, which is why low carbon dioxide concentrations in the growth medium often present a bottleneck that impedes rapid biomass growth [62]. Therefore, additional CO2 needs to be loaded into the photobioreactor by increasing saturation or enriching the culture with leachate from the digesters [63]. Some promising studies on carbon dioxide fixation in algae cultivation systems indicate that the technology may potentially be used to lower CO2 emissions [60][63].
One advantage of intensive algae production systems is that microalgal biomass can be grown in both freshwater and saltwater media. Kuei-Ling and Jo-Shu (2012) examined Chlorella vulgaris ESP-31 growth in freshwater using a modified Bristol’s medium and MBL medium, producing biomass concentrations of 2.0–5.0 g dry matter·dm−3 for both media [64]. In another study involving Nannochloropsis salina CCAP849 grown in saltwater and F/2 medium, Beacham et al. (2015) obtained a final microalgal cell concentration of 7 × 107 cell·cm−3 [65]. Unlike terrestrial plants, microalgae do not require fertile farmland to thrive [45][66] and can live, effectively photosynthesize, and build biomass in various climate conditions [41].
Eutrophic and degraded water bodies can be used as another promising source of microalgal biomass [67][68]. Extracting microalgae from such reservoirs leads to a direct improvement in water quality [43][69]. Microalgae blooms, particularly cyanobacteria blooms, pose a threat to regions attractive to tourists and disrupt the basic processes of natural water bodies [70]. For example, Lake Taihu in China, a source of potable water for over two million people, has been repeatedly struck by cyanobacteria blooms since 2007, impacting water quality and posing a technological challenge concerning water treatment [71]. Some researchers have attempted to use microalgae from Lake Taihu as an organic substrate for biogas production [71][72]. Microalgal blooms, most of which are cyanobacteria blooms, are increasingly occurring in water bodies worldwide. Lake Chaohu and Lake Dianchi are among the reservoirs that regularly experience algal blooms [73].
Controlled cultivation of microalgae in eutrophic sea waters has been shown to directly lower biogenic compound concentration in the water and reduce the likelihood of marine life loss. Thus, it can be viewed as a method of revegetation used to improve reservoir condition [57]. Some of the associated issues were addressed in a research program launched by the present authors, which in large part aimed to assess the potential of incorporating microalgal biomass sourced from the Lagoon of Wisła and microalgae sourced from the Puck Bay into methane fermentation processes [74][75][76]. The analysis of the microalgae sourced from the Lagoon of Wisła showed a taxonomically differentiated biomass undergoing season-to-season changes. Bacillariophyceae species prevailed in the spring months from April to May and in the autumn months from October to November. From June to September, the Cyanoprokaryota division species were the most populous, with Chlorophyta and Dinophyceae as the subdominant groups [70]. It was shown that the time of microalgae extraction from Lagoon of Wisła waters had a significant effect on the organic compound concentration in phytoplankton dry matter. The lowest concentrations were recorded for the Bacillariophyceae-dominant period, whereas the highest ones were correlated with Cyanoprokaryota and Chlorophyta presence [262]. Respirometric analyses showed that the technological performance of the methane fermentation process was the highest in the variants utilizing algal biomass extracted between June and September (i.e., rich in Cyanoprokaryota and, to a lesser extent, Chlorophyta) loaded into model digesters. Biogas yields within this period ranged between 389.07 ± 8.21 and 420.95 ± 0.95 cm3·g dry matter−1 [70].
Microalgae can be grown in water sourced from natural reservoirs (with a high content of biogenic substances), as well as in liquid waste and wastewater of various compositions. The use of such culture media not only leads to increased biomass productivity but can also deliver positive environmental outcomes. Microalgae employed in a photobioreactor with a scrubber allowed for a 60–90% reduction in nitrogen content and 70–100% reduction in phosphorus content in an effluent from manure condensation [77]. Microalgae-based technological systems also offer the advantage of pesticide-free cultivation, which significantly reduces the risk of secondary environmental pollution[41].
The reservations and controversies surrounding microalgae production/utilization technology mostly relate to the identified investment, technological, and operational barriers to implementation. Such barriers directly impact the costs of biomass cultivation, thickening, and separation. Another dissuading factor is the financial burden connected with converting the biomass into valuable end products [41][42]. The investment and operating costs intrinsic to microalgal cultivation are several times higher (more than tenfold in some cases) than the costs of extracting lignocellulosic biomass [49][78]. As such, the priority task of commercial enterprises and research groups is to increase the cost-effectiveness of such systems [39]. Furthermore, operating microalgae production installations and converting biomass into other products are still subject to many technological hurdles [66]. Gouveia (2011) noted the multiple deficiencies of algae cultivation methods, pointing to the recurring problems with growing microalgae in photobioreactors, i.e., biofilm build-up on photobioreactor walls, blockage of light sources by the growing culture, high oxygen concentrations, and accumulation of compounds toxic to microalgae cells [79]. Other authors also highlighted the importance of these technological problems [80][81].
In order to obtain economically profitable, pure cultures and metabolites of microalgae, it is necessary to employ complex substrate compositions, containing nitrogen, phosphorus, iron, silicon, vitamins, and microelements [43][66]. Operators of intensive microalgae production systems face the major technological challenge of ensuring proper composition of the growth medium and monitoring its quality throughout cultivation. The choice of growth medium depends on the tested microalgae species, as well as on the desired product of cultivation. For example, Nannochloropsis oceanica cultivated for biofuel production is grown on BG-11 medium, at 2% CO2 (v/v), with an artificial light intensity of 80–100 μmol photons·m−2·s−1 and a temperature of 25 °C [82]. In contrast, Crypthecodinium cohnii microalgae grown to produce omega-3 acids need glucose as a source of carbon, yeast extract as a source of nitrogen, a temperature of 27 °C, dark conditions, and oxygen levels of more than 30% [83].
Improper operation of microalgal biomass production systems may lead to problematic environmental pollution with undigested nutrients. This phenomenon causes adverse changes in the functioning and structure of aquatic ecosystems, leading to accelerated eutrophication. The problem stems in large part from bioreactors being fed with an imbalanced nutrient load. The discharge of effluent rich in excess nutrients into natural reservoirs may result in acidification and water pollution, which in turn lead to ecotoxicity, eutrophication, and degradation [69][84].
Other disadvantages of microalgal biomass technologies relate to the potential competition of algae with food crops and industrial crops, land use and the change thereof, and negative effects on biodiversity [66]. Researchers also pointed to potential disruption of natural aquatic ecosystems [85], ozone depletion [86], and structural restrictions on the market’s operation [66]. Additionally, genetically modified microalgae used for cultivation may proliferate in the wild and produce various mutations, including ones detrimental to the environment [69]. The lack of legislative/legal measures and incentives, such as subsidies and tax credits, also presents a barrier to the widespread take-up of microalgae-based technologies, including those relevant to biofuel production [85].
This entry is adapted from the peer-reviewed paper 10.3390/su12239980
References
- Deviram, G.; Mathimani, T.; Anto, S.; Ahamed, T.S.; Ananth, D.A.; Pugazhendhi, A. Applications of microalgal and cyanobacterial biomass on a way to safe, cleaner and a sustainable environment. J. Clean. Prod. 2020, 253, 119770. [Google Scholar] [CrossRef]
- Kamani, M.H.; Eş, I.; Lorenzo, J.M.; Remize, F.; Roselló-Soto, E.; Barba, F.J.; Clark, J.H.; Khaneghah, A.M. Advances in plant materials, food by-products, and algae conversion into biofuels: Use of environmentally friendly technologies. Green Chem. 2019, 21, 3213–3231. [Google Scholar] [CrossRef]
- Patil, S.; Prakash, G.; Lali, A.M. Reduced chlorophyll antenna mutants of Chlorella saccharophila for higher photosynthetic efficiency and biomass productivity under high light intensities. J. Appl. Phycol. 2020, 32, 1559–1567. [Google Scholar] [CrossRef]
- Santhakumaran, P.; Ayyappan, S.M.; Ray, J.G. Nutraceutical applications of twenty-five species of rapid-growing green-microalgae as indicated by their antibacterial, antioxidant and mineral content. Algal Res. 2020, 47, 101878. [Google Scholar] [CrossRef]
- Tolboom, S.N.; Carrillo-Nieves, D.; de Jesús Rostro-Alanis, M.; de la Cruz Quiroz, R.; Barceló, D.; Iqbal, H.M.N.; Parra-Saldivar, R. Algal-based removal strategies for hazardous contaminants from the environment—A review. Sci. Total. Environ. 2019, 665, 358–366. [Google Scholar] [CrossRef] [PubMed]
- Ziolkowska, J.R. Chapter 1—Biofuels technologies: An overview of feedstocks, processes, and technologies. In Biofuels for a More Sustainable Future; Elsevier: Amsterdam, The Netherlands; Oxford, UK; Cambridge, MA, USA, 2020; pp. 1–19. [Google Scholar] [CrossRef]
- Stiles, W.A.V.; Styles, D.; Chapman, S.P.; Esteves, S.; Bywater, A.; Melville, L.; Silkina, A.; Lupatsch, I.; Fuentes, C.; Lovitt, R.; et al. Using microalgae in the circular economy to valorise anaerobic digestate: Challenges and opportunities. Bioresour. Technol. 2018, 267, 732–742. [Google Scholar] [CrossRef] [PubMed]
- SundarRajan, P.; Gopinath, K.P.; Greetham, D.; Antonysamy, A.J. A review on cleaner production of biofuel feedstock from integrated CO2 sequestration and wastewater treatment system. J. Clean. Prod. 2019, 210, 445–458. [Google Scholar] [CrossRef]
- Nawaz, T.; Rahman, A.; Pan, S.; Dixon, K.; Petri, B.; Selvaratnam, T. A review of landfill leachate treatment by microalgae: Current status and future directions. Processes 2020, 8, 384. [Google Scholar] [CrossRef]
- Nagarajan, D.; Lee, D.J.; Chang, J.S. Biogas Upgrading by Microalgae: Strategies and Future Perspectives. In Microalgae Biotechnology for Development of Biofuel and Wastewater Treatment; Alam, M., Wang, Z., Eds.; Springer: Singapore, 2019. [Google Scholar] [CrossRef]
- Rahman, A.; Agrawal, S.; Nawaz, T.; Pan, S.; Selvaratnam, T. A review of algae-based produced water treatment for biomass and biofuel production. Water 2020, 12. 2351. [Google Scholar] [CrossRef]
- Sahu, S.K.; Mantri, V.A.; Zheng, P.; Yao, N. Chapter 1 Algae Biotechnology. Current Status, Potential and Impediments. In Encyclopedia of Marine Biotechnology; John Wiley & Sons Ltd: Hoboken, NJ, USA, 2020. [Google Scholar] [CrossRef]
- Ganesan, R.; Manigandan, S.; Samuel, M.S.; Shanmuganathan, R.; Brindhadevi, K.; Chi, N.T.L.; Duc, P.A.; Pugazhendhi, A. A review on prospective production of biofuel from microalgae. Biotechnol. Rep. 2020, 27, e00509. [Google Scholar] [CrossRef]
- Peng, L.; Fu, D.; Chu, H.; Wang, Z.; Qi, H. Biofuel production from microalgae: A review. Environ. Chem. Lett. 2020, 18, 285–297. [Google Scholar] [CrossRef]
- Coimbra, R.N.; Escapa, C.; Otero, M. Comparative Thermogravimetric Assessment on the Combustion of Coal, Microalgae Biomass and Their Blend. Energies 2019, 12, 2962. [Google Scholar] [CrossRef]
- Panahi, H.K.S.; Tabatabaei, M.; Aghbashlo, M.; Dehhaghi, M.; Rehan, M.; Nizami, A.-S. Recent updates on the production and upgrading of bio-crude oil from microalgae. Bioresour. Technol. Rep. 2019, 7, 100216. [Google Scholar] [CrossRef]
- Feng, R.; Zaidi, A.A.; Zhang, K.; Shi, Y. Optimization of microwave pretreatment for biogas enhancement through anaerobic digestion of microalgal biomass. Period. Polytech. Chem. Eng. 2018, 63, 65–72. [Google Scholar] [CrossRef]
- Córdova, O.; Chamy, R. Chapter 15—Microalgae to Biogas: Microbiological Communities Involved. In Microalgae Cultivation for Biofuels Production; Elsevier: London, UK; San Diego, CA, USA; Oxford, UK, 2020; pp. 227–249. [Google Scholar] [CrossRef]
- Li, F.; Hülsey, M.J.; Yan, N.; Dai, Y.; Wang, C.-H. Co-transesterification of waste cooking oil, algal oil and dimethyl carbonate over sustainable nanoparticle catalysts. Chem. Eng. J. 2020, 405, 127036. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yang, H.; Zhang, X.; Han, F.; Tu, W.; Yang, W. Microalgal Hydrogen Production. Small Methods 2020, 4. [Google Scholar] [CrossRef]
- Özçimen, D.; Koçer, A.T.; İnan, B.; Özer, T. Chapter 14—Bioethanol production from microalgae. In Handbook of Microalgae-Based Processes and Products; Elsevier: London, UK; San Diego, CA, USA; Cambridge, MA, USA; Oxford, UK, 2020; pp. 373–389. [Google Scholar] [CrossRef]
- Chernova, N.I.; Kiseleva, S.V.; Larina, O.M.; Sytchev, G.A. Manufacturing gaseous products by pyrolysis of microalgal biomass. Int. J. Hydrog. Energy 2019, 45, 1569–1577. [Google Scholar] [CrossRef]
- Lee, X.J.; Ong, H.C.; Gan, Y.Y.; Chen, W.-H.; Mahlia, T.M.I. State of art review on conventional and advanced pyrolysis of macroalgae and microalgae for biochar, bio-oil and bio-syngas production. Energy Convers. Manag. 2020, 210. [Google Scholar] [CrossRef]
- Sialve, B.; Bernet, N.; Bernard, O. Anaerobic digestion of microalgae as a necessary step to make microalgal biodiesel. Sustainable. Biotechnol. Adv. 2009, 27, 409–416. [Google Scholar] [CrossRef]
- Campbell, J.; Lobell, D.; Field, C. Greater transportation energy and GHG offsets from bioelectricity than ethanol. Science 2009, 324, 1055–1057. [Google Scholar] [CrossRef] [PubMed]
- Börjesson, P.; Berglund, M. Environmental systems analysis of biogas systems -part I: Fuel-cycle emissions. Biomass Bioenergy 2006, 30, 469–485. [Google Scholar] [CrossRef]
- Ubando, A.T.; Rivera, D.R.T.; Chen, W.H.; Culaba, A.B. A comprehensive review of life cycle assessment (LCA) of microalgal and lignocellulosic bioenergy products from thermochemical processes. Bioresour. Technol. 2019, 291, 121837. [Google Scholar] [CrossRef]
- Ubando, A.T.; Felix, C.B.; Chen, W.-H. Biorefineries in circular bioeconomy: A comprehensive review. Bioresour. Technol. 2020, 299, 122585. [Google Scholar] [CrossRef] [PubMed]
- Chandra, R.; Iqbal, H.M.N.; Vishal, G.; Lee, H.S.; Nagra, S. Algal biorefinery: A sustainable approach to valorize algal-based biomass towards multiple product recovery. Bioresour. Technol. 2019, 278, 346–359. [Google Scholar] [CrossRef] [PubMed]
- Kisielewska, M.; Zieliński, M.; Dębowski, M.; Kazimierowicz, J.; Romanowska-Duda, Z.; Dudek, M. Effectiveness of Scenedesmus sp. Biomass Grow and Nutrients Removal from Liquid Phase of Digestates. Energies 2020, 13, 1432. [Google Scholar] [CrossRef]
- Mishra, S.; Roy, M.; Mohanty, K. Microalgal bioenergy production under zero-waste biorefinery approach: Recent advances and future perspectives. Bioresour. Technol. 2019, 292, 122008. [Google Scholar] [CrossRef]
- Metsoviti, M.N.; Papapolymerou, G.; Karapanagiotidis, I.T.; Katsoulas, N. Comparison of Growth Rate and Nutrient Content of Five Microalgae Species Cultivated in Greenhouses. Plants 2019, 8, 279. [Google Scholar] [CrossRef]
- Aziz, M.M.A.; Kassim, A.K.; Shokravi, Z.; Jakarni, F.M.; Liu, H.Y.; Zaini, N. Two-stage cultivation strategy for simultaneous increases in growth rate and lipid content of microalgae: A review. Renew. Sustain. Energy Rev. 2020, 119, 109621. [Google Scholar] [CrossRef]
- Sánchez-Bayo, A.; Morales, V.; Rodríguez, R.; Vicente, G.; Bautista, L.F. Cultivation of microalgae and cyanobacteria: Effect of operating conditions on growth and biomass composition. Molecules 2020, 25, 2834. [Google Scholar] [CrossRef] [PubMed]
- Neofotis, P.; Huang, A.; Sury, K.; Chang, W.; Joseph, F.; Gabr, A.; Twary, S.; Qiu, W.; Holguine, O.; Polle, J.E.W. Characterization and classification of highly productive microalgae strains discovered for biofuel and bioproduct generation. Algal Res. 2016, 15, 164–178. [Google Scholar] [CrossRef]
- Li, P.; Sakuragi, K.; Makino, H. Extraction Techniques in Sustainable Biofuel Production: A Concise Review. Fuel Process. Technol. 2019, 193, 295–303. [Google Scholar] [CrossRef]
- Piasecka, A.; Nawrocka, A.; Wiącek, D.; Krzemińska, J. Agro-industrial by-product in photoheterotrophic and mixotrophic culture of Tetradesmus obliquus: Production of ω3 and ω6 essential fatty acids with biotechnological importance. Sci Rep. 2020, 10, 1–11. [Google Scholar] [CrossRef]
- Chen, W.-H.; Lin, B.-J.; Huang, M.-Y.; Chang, J.-S. Thermochemical conversion of microalgal biomass into biofuels: A review. Bioresour. Technol. 2015, 184, 314–327. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, J.; Aila, M.; Bangwal, D.P.; Kaul, S.; Garg, M.O. Algae based biorefinery—How to make sense? Renew. Sustain. Energy Rev. 2015, 47, 295–307. [Google Scholar] [CrossRef]
- Dębowski, M.; Zieliński, M. The possibility of using algal cultures in wastewater treatment processes. Water Sewage 2009, 9, 9–12. [Google Scholar]
- Bharathiraja, B.; Chakravarthy, M.; Kumar, R.R.; Yogendran, D.; Yuvaraj, D.; Jayamuthunagai, J. Aquatic biomass (algae) as a future feed stock for bio-refineries: A review on cultivation, processing and products. Renew. Sustain. Energy Rev. 2015, 47, 634–653. [Google Scholar] [CrossRef]
- Ullah, K.; Ahmad, M.; Sofia Sharma, V.K.; Lu, P.; Harvey, A.; Zafar, M. Assessing the potential of algal biomass opportunities for bioenergy industry: A review. Fuel 2015, 143, 414–423. [Google Scholar] [CrossRef]
- Sambusiti, C.; Bellucci, M.; Zabaniotou, A.; Beneduce, L.; Monlau, F. Algae as promising feedstocks for fermentative biohydrogen production according to a biorefinery approach: A comprehensive review. Renew. Sustain. Energy Rev. 2015, 44, 20–36. [Google Scholar] [CrossRef]
- Schenk, P.M.; Thomas-Hall, S.R.; Stephens, E.; Marx, U.C.; Mussgnug, J.H. Second generation biofuels: High-efficiency microalgae for biodiesel production. Bioenergy Res. 2008, 1, 20–43. [Google Scholar] [CrossRef]
- Raheem, A.; Wan Azlina, W.A.K.G.; Taufiq Yap, Y.H.; Danquah, M.K.; Harun, R. Thermochemical conversion of microalgal biomass for biofuel production. Renew. Sustain. Energy Rev. 2015, 49, 990–999. [Google Scholar] [CrossRef]
- Tredici, M.R.; Bassi, N.; Prussi, M.; Biondi, N.; Rodolfi, L.; Chini Zittelli, G.; Sampietro, G. Energy balance of algal biomass production in a 1-ha ‘‘Green Wall Panel’’ plant: How to produce algal biomass in a closed reactor achieving a high Net Energy Ratio. Appl. Energy 2015, 154, 1103–1111. [Google Scholar] [CrossRef]
- Maxwell, D.P.; Falk, S.; Trick, C.G.; Huner, N.P.A. Growth at low temperature mimics high-light acclimation in Chlorella vulgaris. Plant Physiol. 1994, 105, 535–543. [Google Scholar] [CrossRef] [PubMed]
- Raslavicius, L.; Semenov, V.G.; Chernova, N.I.; Kersys, A.; Kopeyka, A.K. Producing transportation fuels from algae: In search of synergy. Renew. Sustain. Energy Rev. 2014, 40, 133–142. [Google Scholar] [CrossRef]
- Huber, G.W.; Iborra, S.; Corma, A. Synthesis of transportation fuels from biomass: Chemistry, catalysts, and engineering. Chem. Rev. 2006, 106, 4044–4098. [Google Scholar] [CrossRef]
- Hong, W.; Yu, A.; Heo, S.; Oh, B.; Kim, C.; Sohn, J.; Yang, J.W.; Kondo, A.; Seo, J.W. Production of lipids containing high levels of docosahexaenoic acid from empty palm fruit bunches by Aurantiochytrium sp. KRS101. Bioprocess. Biosyst. Eng. 2013, 36, 959–963. [Google Scholar] [CrossRef] [PubMed]
- Oswald, W.J. Ponds in the twenty-first century. Water Sci. Technol. 1995, 31, 1–8. [Google Scholar] [CrossRef]
- Grobbelaar, J.U. Physiological and technological considerations for optimising mass algal cultures. J. Appl. Phycol. 2000, 12, 201–206. [Google Scholar] [CrossRef]
- Mùnoz, R.; Kollner, C.; Guieysse, B.; Mattiasson, B. Photosynthetically oxygenated salicylate biodegradation in a continuous stirred tank photobioreactor. Biotechnol. Bioeng. 2004, 87, 797–803. [Google Scholar] [CrossRef]
- Oswald, W.J. My sixty years in applied algology. J. Appl. Phycol. 2003, 15, 99–106. [Google Scholar] [CrossRef]
- Javed, F.; Aslam, M.; Rashid, N.; Shamair, Z.; Khan, A.L.; Yasin, M.; Fazal, T.; Hafeez, A.; Rehman, F.; Rehman, M.S.U.; et al. Microalgae-based biofuels, resource recovery and wastewater treatment: A pathway towards sustainable biorefinery. Fuel 2019, 255, 115826. [Google Scholar] [CrossRef]
- van Harmelen, T.; Oonk, H. Microalgae Biofixation Processes: Applications and Potential Contributions to Greenhouse Gas Mitigation Options; International Network on Biofixation of CO2 and Greenhouse Gas Abatement with Microalgae: Apeldoom, The Netherlands, 2006. [Google Scholar]
- Wang, B.; Li, Y.; Wu, N.; Lan, C. CO2 bio-mitigation using microalgae. Appl. Microbiol. Biotechnol. 2008, 79, 707–718. [Google Scholar] [CrossRef]
- Molazadeh, M.; Ahmadzadeh, H.; Pourianfar, H.R.; Lyon, S.; Rampelotto, P.H. The Use of Microalgae for Coupling Wastewater Treatment with CO2 Biofixation. Front. Bioeng. Biotechnol. 2019, 7, 42. [Google Scholar] [CrossRef] [PubMed]
- Lundquist, T.J. Production of algae in conjunction with wastewater treatment. In NREL—AFOSR Workshop on Algal Oil for Jet Fuel Production; Arlington, VA, USA, 2008; Available online: https://www.researchgate.net/profile/John_Benemann2/publication/229039925_Overview_Algae_oil_to_biofuels/links/54171cbf0cf2218008bed0aa/Overview-Algae-oil-to-biofuels.pdf (accessed on 8 August 2020).
- Jacob-Lopes, E.; Scoparo, C.H.G.; Queiroz, M.I.; Franco, T.T. Biotransformations of carbon dioxide in photobioreactors. Energy Convers. Manag. 2010, 51, 894–900. [Google Scholar] [CrossRef]
- Chiu, S.-Y.; Kao, C.-Y.; Tsai, M.-T.; Ong, S.-C.; Chen, C.-H.; Lin, C.-S. Lipid accumulation and CO2 utilization of Nanochloropsis oculata in response to CO2 aeration. Bioresour. Technol. 2009, 100, 833–841. [Google Scholar] [CrossRef] [PubMed]
- Chiu, S.-Y.; Kaom, C.-J.; Chen, C.-H.; Kuan, T.-C.; Ong, S.-C.; Lin, C.-S. Reduction of CO2 by a high-density culture of Chlorella sp. in a semicontinuous photobioreactor. Bioresour. Technol. 2008, 99, 3389–3396. [Google Scholar] [CrossRef]
- De Morais, M.G.; Costa, J.A.V. Isolation and selection of microalgae from coal fired thermoelectric power plant for biofixation of carbon dioxide. Energy Convers. Manag. 2007, 48, 2169–2173. [Google Scholar] [CrossRef]
- Kuei-Ling, Y.; Jo-Shu, C. Effects of cultivation conditions and media composition on cell growth and lipid productivity of indigenous microalga Chlorella vulgaris ESP-31. Bioresour. Technol. 2012, 105, 120–127. [Google Scholar]
- Beachama, T.A.; Mora Maciaa, V.; Rooks, P.; White, D.A.; Ali, S.T. Altered lipid accumulation in Nannochloropsis salina CCAP849/ 3 following EMS and UV induced mutagenesis. Biotech. Rep. 2015, 7, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Noraini, M.Y.; Ong, H.C.; Badrul, M.J.; Chong, W.T. A review on potential enzymatic reaction for biofuel production from algae. Renew. Sustain. Energy Rev. 2014, 39, 24–34. [Google Scholar] [CrossRef]
- Dębowski, M.; Zieliński, M.; Dudek, M.; Grala, A. Acquisition feasibility and methane fermentation effectiveness of biomass of microalgae occurring in eutrophicated aquifers on the example of The Vistula Lagoon. Int. J. Green Energy 2016, 13, 395–407. [Google Scholar] [CrossRef]
- Dębowski, M.; Zieliński, M.; Kisielewska, M.; Krzemieniewski, M. Efficiency of methane fermentation of waste microalgae biomass (WMAB) collected in processes of reclamation of eutrophicated water reservoirs. Environ. Earth Sci. 2016, 75, 525. [Google Scholar] [CrossRef]
- Slade, R.; Bauen, A. Micro-algae cultivation for biofuels: Cost, energy balance, environmental impacts and future prospects. Biomass Bioenergy 2013, 53, 29–38. [Google Scholar] [CrossRef]
- Dębowski, M. The Use of Algae Biomass as a Substrate in the Methane Fermentation Process; UWM: Olsztyn, Poland, 2013. [Google Scholar]
- Guo, L. Doing battle with the green monster of Taihu Lake. Science 2007, 317, 1166. [Google Scholar] [CrossRef]
- Zhong, W.; Zhang, Z.; Luo, Y.; Qiao, W.; Xiao, M.; Zhang, M. Biogas productivity by co-digesting Taihu blue algae with corn straw as an external carbon source State Key. Bioresour. Technol. 2012, 114, 281–286. [Google Scholar] [CrossRef]
- Qin, B. Lake eutrophication: Control countermeasures and recycling exploitation. Ecol. Eng. 2009, 35, 1569–1573. [Google Scholar] [CrossRef]
- Dębowski, M.; Grala, A.; Zieliński, M.; Dudek, M. Efficiency of the methane fermentation process of macroalgae biomass originating from puck bay. Arch. Environ. Prot. 2012, 38, 99–107. [Google Scholar]
- Dębowski, M.; Zieliński, M.; Rokicka, M.; Kupczyk, K. The possibility of using macroalgae biomass from natural reservoirs as a substrate in the methane fermentation process. Int. J. Green Energy 2015, 12, 970–977. [Google Scholar] [CrossRef]
- Dębowski, M.; Zieliński, M.; Kupczyk, K.; Rokicka, M.; Hajduk, A. Effect of taxonomic diversification of microalgae harvested from eutrophicated reservoirs on the chemical composition of biomass and effectiveness of methane fermentation. Environ. Prog. Sustain. Energy 2015, 34, 858–865. [Google Scholar] [CrossRef]
- AquaFUELs. Report on Biology and Biotechnology of Algae with Indication of Criteria for Strain Selection. 2011. Available online: http://www.aquafuels.eu/deliverables.html (accessed on 8 August 2020).
- Ryan, D.; Jennifer, M.; Christopher, K.; Nicholas, G.; Eric, T. Process Design and Economics for the Production of Algal Biomass: Algal Biomass Production in Open Pond Systems and Processing Through Dewatering for Downstream Conversion; Technical Report NREL/TP-5100-64772; National Renewable Energy Laboratory: Golden, CO, USA, 2016; pp. 1–119. [Google Scholar]
- Gouveia, L. Microalgae as a Feedstock for Biofuels; Springer: Berlin/Heidelberg, Germany; Dordrecht, The Netherlands; London, UK; New York, NY, USA, 2011. [Google Scholar]
- Mustapa, N.S.; Abu Mansor, M.S.; Serri, N.A. Design and development of centred-light photobioreactor for microalgae cultivation system. IOP Conf. Ser. Mater. Sci. Eng. 2020, 716, 012009. [Google Scholar] [CrossRef]
- Rebolledo-Oyarce, J.; Mejía-López, J.; García, G.; Rodríguez-Córdova, L.; Sáez-Navarrete, C. Novel photobioreactor design for the culture of Dunaliella tertiolecta—Impact of color in the growth of microalgae. Bioresour. Technol. 2019, 289, 121645. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Wang, Z.; Zhu, M.; Yu, C.; Cao, Y.; Zhang, D.; Zhou, G. Increased lipid productivity and TAG content in Nannochloropsis by heavy-ion irradiation mutagenesis. Bioresour. Technol. 2013, 136, 360–367. [Google Scholar] [CrossRef] [PubMed]
- De Swaaf, M.; Sijtsma, L.; Pronk, J. High-cell-density fed-batch cultivation of the docosahexaenoic acid producing microalga Crypthecodinium cohnii. Biotechnol. Bioeng. 2003, 81, 666–672. [Google Scholar] [CrossRef] [PubMed]
- Lill, J.-O.; Salovius-Lauren, S.; Harju, L.; Rajander, J.; Saarela, K.-E.; Lindroos, A. Temporal changes in elemental composition in decomposing filamentous algae (Cladophora glomerata and Pilayella littoralis) determined with PIXE and PIGE. Sci. Total. Environ. 2012, 414, 646–652. [Google Scholar] [CrossRef]
- Ziolkowska, J.R.; Simon, L. Recent developments and prospects for algae-based fuels in the US. Renew. Sustain. Energy Rev. 2014, 29, 847–853. [Google Scholar] [CrossRef]
- Singh, A.; Nigam, P.S.; Murphy, J.D. Renewable fuels from algae: An answer to debatable land based fuels. Bioresour. Technol. 2011, 102, 10–16. [Google Scholar] [CrossRef]
- Lill, J.-O.; Salovius-Lauren, S.; Harju, L.; Rajander, J.; Saarela, K.-E.; Lindroos, A. Temporal changes in elemental composition in decomposing filamentous algae (Cladophora glomerata and Pilayella littoralis) determined with PIXE and PIGE. Sci. Total. Environ. 2012, 414, 646–652. [Google Scholar] [CrossRef]
- Ziolkowska, J.R.; Simon, L. Recent developments and prospects for algae-based fuels in the US. Renew. Sustain. Energy Rev. 2014, 29, 847–853. [Google Scholar] [CrossRef]
- Singh, A.; Nigam, P.S.; Murphy, J.D. Renewable fuels from algae: An answer to debatable land based fuels. Bioresour. Technol. 2011, 102, 10–16. [Google Scholar] [CrossRef]