Ascorbic acid (ASC) is a key nutrient that serves as an antioxidant and a cofactor for numerous enzymatic reactions. However, humans, unlike most mammals, are unable to synthesize it. Consequently, ASC must be obtained through dietary sources, especially fresh fruit and vegetables. The value of administering exogenous micronutrients, to reestablish antioxidant concentrations in patients with severe disease, has been recognized for decades. Despite the suggestion that ASC supplementation may reduce oxidative stress and prevent several chronic conditions, few large, randomized clinical trials have tested it in patients with severe illness. This article reviews the recent literature on the pharmacological profile of ASC and the role of its supplementation in critically ill patients.
- Vitamin C, ascorbic acid, antioxidant
1. Introduction
Vitamin C, also known as ascorbic acid (ASC), is a key antioxidant, a cofactor in essential enzyme reactions, and a key nutrient.
In several animals, it is synthesized in the liver or kidneys, whereas some species, such as humans and non-human primates, have lost this ability due to mutations in the coding sequence of the last committed enzyme of the pathway and must obtain it from the diet [1]. The form of ASC found in dietary sources is l-threo-hex-2-enono-1,4-lactone, which was originally called hexuronic acid. When, in the early 1930s, Szent-Györgyi identified and isolated it as the molecule capable of preventing and treating scurvy, it was renamed ascorbate. Scurvy typically developed during long sea voyages, weeks after the provisions of fresh fruit and vegetables had run out [2]. Failure to treat it led to death. Seamen discovered that it could be treated and prevented by citrus fruit, even though this lay remedy was scorned by physicians as well as scientists.
The enzymatic reactions for which it is a cofactor involve dioxygenases, which participate in a wide range of physiological processes including the synthesis of collagen, carnitine, norepinephrine, and serotonin;the regulation of hypoxia-inducible transcription factor (HIF); and histone demethylation [3][4].
ASC is an essential component of the human diet, found in a wide range of food products, especially fresh vegetables and fruit [5]. In adults, the recommended daily dose of around 100 mg has been demonstrated to ensure a plasma concentration of 50 M. Since ASC performs most of its functions inside cells, it is transported through their plasma membranes. ASC exists in two molecular forms thatare characterized by different chemical stabilities, half-lives in vivo, and transport mechanisms. The oxidized form, dehydroascorbic acid (DHA), is transported from the extracellular medium into the cell by glucose transporters (GLUTs), whereas the reduced form, ASC, is transported by sodium–ASC co-transporters (SVCTs) [6][7].
ASC also plays a well-documented therapeutic role in conditions such as cancer, cardiovascular disease (CVD), and infectious disorders; however, rigorous clinical trials are too few to allow drawing any conclusions, especially where cancer is concerned.
2. ASC Pharmacokinetics
The pharmacokinetics of orally administered ASC are non-linear and differ widely from thoseof most low-molecular-weight drugs [8]. However, scanty information hampers the interpretation of the results of clinical studies [8][9][10]. The pharmacokinetics of ASC aredetailed below.
2.1. ASC Absorption
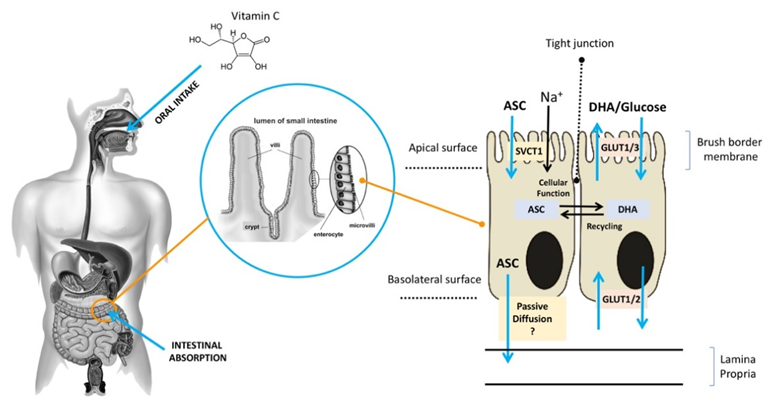
ASC is mainly obtained from dietary sources, especially vegetables and fruit [11]. Diets rich in ASC provide an adequate amount of the vitamin for healthy individuals [12], but they may be insufficient for those who suffer from chronic ASC insufficiency due to lifestyle habits (smokers) or to disease (scurvy) [8][13][14]. ASC is found in a reduced (ASC) and an oxidized (DHA) form [15]. It is highly water-soluble, and more than 99.9% is available in an anionic form at a pH of 7.0, which may slow down its diffusion rate across the plasma membrane even in the presence of a significant concentration gradient. In acidic environments, ASC is commonly found in an un-ionized form, which facilitates its absorption, largely through passive diffusion. After the oral administration of ASC, individuals with normal levels of ASC have similar times to maximal plasma concentration, although it is unclear whether passive diffusion contributes significantly to its absorption through this route [16][17] (Figure 1). Active transport plays a key role in ASC absorption independently of the concentration gradient. As early as the 1970s, ASC bioavailability was reported to be highly dose-dependent [18][19][20][21]. Increasing oral doses were found to be associated with decreasing absorption rates, and several studies reported that intestinal ASC absorption is a function of saturable active transport [22][23]. As shown in Figure 1, after oral intake, ASC is mostly absorbed by brush border membrane cells of the intestinal epithelium. In the gut, ASC and DHA are taken up by distinct mechanisms [24][25]. ASC absorption is sodium-dependent and is mediated by SVCT1, a sodium-coupled active transporter [16][18]. The SVCT family was discovered by Tsukaguchi and co-workers, who identified the low-affinity/high-capacity active transporter SVCT1 in the intestine [7]. SVCT1 is also expressed in the epithelium of the proximal renal tubule, where it is responsible for active ASC resorption in the kidney [16]. Indeed, in Slc23a1−/− mice, which lack SVCT1, the renal fractional excretion of ASC is increased by up to 18 times, whereas its intestinal absorption is not significantly reduced [25]. These findings support the notion that renal SVCT1-mediated resorption is crucial for ASC homeostasis. The action of SVCT1 is dose-dependent, and in several tissues, its expression seems to be modulated by ASC concentrations [26]. Although the mechanisms responsible for ASC efflux to plasma are still unclear, volume-sensitive anion channels are conceivably involved [19]. As regards DHA, its absorption is mediated by GLUT1 or GLUT3; as a consequence, DHA competes with glucose for transport, and its uptake is hampered by excess glucose; nonetheless, in the absence of glucose, ASC and DHA show similar maximal uptakes [18]. DHA transport to the circulation through the basolateral membrane and its transformation to ASC in cells may contribute to DHA uptake by keeping the intracellular concentrations of ASC at a low level [20][21]. In sum, ASC is easily carried across the apical membrane of intestinal epithelial cells by active transport, whereas the mechanisms involved in its efflux to the circulation are still unclear. Although intracellular ASC is efficiently kept reduced, thus facilitating further DHA uptake, DHA efflux to the circulation through the GLUTs does not play a significant role. Since the neutral intracellular pH involves a predominance of the anionic form (99.9%), the hydrophilicity of ASC results in fairly slow passive efflux [27]. These findings suggest the presence of yet-unknown transporters/channels that facilitate ASC efflux.
Figure 1. Absorption of ASC across the brush border membrane in intestinal epithelial cells (ASC: ascorbic acid;DHA: dehydroascorbic acid;SVCT: sodium–ASC co-transporters;GLUT: glucose transporters).
2.2. ASC Distribution
The distribution of ASC is highly compartmentalized. Notably, increasing intake does not raise steady-state plasma concentrations beyond 70–80 µM [17]. A daily intake of 200–400 mg results in plasma saturation [28]. In some individuals (e.g., smokers) and conditions (e.g., pregnancy and a variety of diseases), a higher intake is required to maintain a sufficient concentration. However, its transport across membranes, at least its distribution from blood to tissues, is unlikely to be mainly achieved through simple diffusion. In healthy individuals, intracellular ASC concentrations range from about 0.5 to 10 mM, compared to a mere 50–80 μM in the plasma [29]. The low plasma concentrations of oxidized ASC (DHA) found in healthy subjects exclude GLUT-mediated transport and play a key role in ASC distribution. The molecular mechanisms underpinning the widely different steady-state concentrations of the vitamin found in different tissues are largely unknown. Until additional tissue-specific SVCT2 isoforms are identified, the steady-state concentrations of ASC in the various organs may be ascribed to SVCT2 levels in cells and to ASC plasma concentrations.
2.3. ASC Metabolism
The metabolism of ASC is closely associated withits redox status. ASC is a chain-breaking antioxidant that quenches free radicals and donates electrons to a large number of mono- and dioxygenases [30][31]. Moreover, a variety of mechanisms ensure that most oxidized ASC is recovered by intracellular recycling and converted back to ASC, its biologically active reduced form, by several cell types [32]. Since in humans, the daily ASC turnover is only 3% [33], the close regulation of its daily intake is essential to maintain a sufficient level. DHA conversion to ASC correlates with dose-dependent renal reuptake and is involved in preserving ASC homeostasis in the body [33][34][35][36][37][38].
2.4. ASC Excretion and Resorption
Due to its low molecular weight and high hydrophilicity, ASC is efficiently excreted through the kidneys. It is mostly filtered through the glomerulus by virtue of the hydrostatic pressure gradient and is concentrated in pre-urine after water resorption. Its increase from <0.01% in plasma to about 15% in pre-urine represents a concentration gradient of 1500:1. Reuptake in the proximal renal tubules is mediated by saturable active transport via SVCT1. The excretion of excess ASC in individuals with plasma saturation can be quantified [17]. In subjects with ASC deficiency, resorption is mainly performed by SVCT1 in the apical membrane, although diffusion from the luminal surface may also contribute to the overall uptake. The renal reuptake of ASC is highly concentration-dependent. Its renal excretion coefficient ranges from 0 to 1, depending on the individual’s ASC concentration; this confirms its predominant reuptake in individuals with ASC deficiency and its predominant excretion in those with saturation [17][35]. In healthy individuals with a daily intake >500 mg, the value of the coefficient is 1, suggesting a minor role for passive renal resorption [17][35].
This entry is adapted from the peer-reviewed paper 10.3390/antiox9121182
References
- Drouin, G.; Godin, J.; Page, B. The Genetics of ASC Loss in Vertebrates. Genom. 2011, 12, 371–378.
- Baron, J. Sailors’ scurvy before and after James Lind—A reassessment. Rev. 2009, 67, 315–332.
- Verrax, J.; Buc Calderon, P. The controversial place of Vitamin C in cancer treatment. Pharmacol. 2008, 76, 1644–1652.
- Du, J.; Yuan, Y.; Si, T.; Lian, J.; Zhao, H. Customized optimization of metabolic pathways by combinatorial transcriptional engineering. Nucleic Acids Res. 2012, 40, e142–e142.
- Rivas, M.; Cobreros, L.; Zeidler, M.; Hombría, J. Plasticity of Drosophila Stat DNA binding shows an evolutionary basis for Stat transcription factor preferences. EMBO Rep. 2008, 9, 1114–1120.
- Vera, A.; Simon, H. Situated Action: A Symbolic Interpretation. Cognitive Science 1993, 17, 7–48.
- Tsukaguchi, H.; Tokui, T.; Mackenzie, B.; Berger, U.; Chen, X.; Wang, Y.; Brubaker, R.; Hediger, M. A family of mammalian Na+-dependent L-ascorbic acid transporters. Nature 1999, 399, 70–75.
- Tveden-Nyborg, P.; Lykkesfeldt, J. Does ASC Deficiency Increase Lifestyle-Associated Vascular Disease Progression? Evidence Based on Experimental and Clinical Studies. Antioxidants & Redox Signaling, 2013 19, 2084–2104.
- Lykkesfeldt, J. and Poulsen, H. Is ASC supplementation beneficial? Lessons learned from randomised controlled trials. J. Nutr., 2009, 103, 1251–1259.
- Frei, B.; Birlouez-Aragon, I.; Lykkesfeldt, J. Authors’ Perspective: What is the Optimum Intake of ASC in Humans? Critical Reviews in Food Science and Nutrition 2012., 52, 815–829.
- Frikke-Schmidt, H.; Tveden-Nyborg, P.; Lykkesfeldt, J. ASC in human nutrition. In Vitamins in the Prevention of Human Disease; Herrmann, W., Obeid, R., Eds.; De Gruyter: Berlin, Germany, 2011; pp. 323–347.
- Carr, A.; Bozonet, S.; Pullar, J.; Simcock, J. and Vissers, M., A Randomized Steady-State Bioavailability Study of Synthetic versus Natural (Kiwifruit-Derived) ASC. Nutrients, 2013, 5, 3684–3695.
- Dachs, G.U.; Munn, D.G.; Carr, A.C.; Vissers, M.C.; Robinson, B.A. Consumption of ASC is below recommended daily intake in many cancer patients and healthy volunteers in Christchurch. Z. Med. J. 2014, 127, 73–76.
- Lykkesfeldt, J.; Christen, S.; Wallock, L.; Chang, H.; Jacob, R. and Ames, B. Ascorbate is depleted by smoking and repleted by moderate supplementation: A study in male smokers and nonsmokers with matched dietary antioxidant intakes. J. Clin. Nutr. 2000, 71, 530–536.
- Du, J.; Cullen, J. and Buettner, G. Ascorbic acid: Chemistry, biology and the treatment of cancer. Biophys. Acta—Rev. Cancer 2012, 1826, 443−457.
- Wang, Y.; Mackenzie, B.; Tsukaguchi, H.; Weremowicz, S.; Morton, C.; Hediger, M. Human ASC (l-Ascorbic Acid) Transporter SVCT1. Biophys. Res. Commun. 2000, 267, 488–494.
- Viscovich, M. ASC pharmacokinetics of plain and slow release formulations in smokers. Nutr. 2004, 23, 1043–1050.
- Malo, C.; Wilson, J. Glucose Modulates ASC Transport in Adult Human Small Intestinal Brush Border Membrane Vesicles. Nutr. 2000, 130, 63–69.
- Lindblad, M.; Tveden-Nyborg, P.; Lykkesfeldt, J. Regulation of ASC Homeostasis during Deficiency. Nutrients 2013, 5, 2860–2879.
- Corpe, C.; Eck, P.; Wang, J.; Al-Hasani, H.; Levine, M. Intestinal Dehydroascorbic Acid (DHA) Transport Mediated by the Facilitative Sugar Transporters, GLUT2 and GLUT8. J. Biol. Chem. 2013, 288, 9092–9101.
- Vera, J.; Rivas, C.; Fischbarg, J.; Golde, D. Mammalian facilitative hexose transporters mediate the transport of dehydroascorbic acid. Nature 1993, 364, 79–82.
- Mayersohn, M. Ascorbic acid absorption in man—pharmacokinetic implications. J. Pharmacol. 1972, 19, 140–142.
- Kubler, W.; Gehler, J. Kinetics of intestinal absorption of ascorbic acid. Calculation of non-dosage-dependent absorption processes. Z. Vitaminforsch. 1970, 40, 442–453.
- Lykkesfeldt J.; Tveden-Nyborg P.; The Pharmacokinetics of Vitamin C. Nutrients. 2019 ,11(10),2412.
- Corpe, et al., ASC transporter Slc23a1 links renal reabsorption, ASC tissue accumulation, and perinatal survival in mice, Clin. Investig. 2010, 120, 1069–1083.
- D. Paidi, et al., Chronic ASC deficiency promotes redox imbalance in the brain but does not alter sodium-dependent ASC transporter 2 expression. Nutrients 2014, 6, 1809–1822.
- SogASCrd, et al., In vivo ASC deficiency in Guinea pigs increases ascorbate transporters in liver but not kidney and brain, Nutr. Res., 2014, 34, 639–645.
- Levine, M.; Conry-Cantilena, C.; Wang, Y.; Welch, R.W.; Washko, P.W.; Dhariwal, K.R.; Park, J.B.; Lazarev, A.; Graumlich, J.F.; King, J.; et al. VitaminC pharmacokinetics in healthy volunteers: Evidence for a recommended dietary allowance. Proc. Natl. Acad. Sci. USA 1996, 93, 3704–3709.
- Frei, B.; Birlouez-Aragon, I.; Lykkesfeldt, J. Authors’ perspective: What is the optimum intake of ASC in humans? Rev. Food Sci. Nutr. 2012, 52, 815–829.
- Hasselholt, S.; Tveden-Nyborg, P.; Lykkesfeldt, J. Distribution of ASC is tissue specific with early saturation of the brain and adrenal glands following differential oral dose regimens in guinea pigs. J. Nutr. 2015, 113, 1539–1549.
- Bode, A.M.; Cunningham, L.; Rose, R.C. Spontaneous decay of oxidized ascorbic acid (dehydro-L-ascorbic acid) evaluated by high-pressure liquid chromatography. Chem. 1990, 36, 1807–1809.
- Lykkesfeldt, J.; Loft, S.; Nielsen, J.B.; Poulsen, H.E. Ascorbic acid and dehydroascorbic acid as biomarkers of oxidative stress caused by smoking. J. Clin. Nutr. 1997, 65, 959–963.
- M. May, Z.C. Qu, R.R. Whitesell, Ascorbic acid recycling enhances the antioxidant reserve of human erythrocytes, Biochemistry 1995, 34, 12721–12728.
- Mardones, L.; Ormazabal, V.; Romo, X.; Jana, C.; Binder, P.; Pena, E.; Vergara, M.; Zuniga, F.A. The glucose transporter-2 (GLUT2) is a low affinity dehydroascorbic acid transporter. Biophys. Res. Commun. 2011, 410, 7–12.
- May, J.M.; Qu, Z.; Morrow, J.D. Mechanisms of ascorbic acid recycling in human erythrocytes. Biophys. Acta 2001, 1528, 159–166.
- Levine, M.; Wang, Y.; Padayatty, S.J.; Morrow, J. A new recommended dietary allowance of ASC for healthy young women. Proc. Natl. Acad. Sci. USA 2001, 98, 9842–9846.
- Recalcati, S.; Gammella, E.; Buratti, P.; Cairo, G. Molecular regulation of cellular Iron balance. IUBMB Life 2017, 69, 389–398.
- Ganz, T.; Nemeth, E. Iron homeostasis in host defence and inflammation. Nat. Rev. Immunol. 2015, 15, 500–510.