Arterial calcifications are present in about 20% of patients with end-stage kidney disease and they reduce the possibility of arterio-venous fistula (AVF) formation and maturation and increase the likelihood of distal ischemia. Arterial assessment is of utmost importance if we are to create distal radiocephalic AVFs in our elderly patients whenever possible without burdening them with futile surgical attempts. A grading system is proposed for quantifying the severity of calcifications in the arteries of the arm with ultrasound exam.
Adapted from [ref].
- arterio-venous fistula
- ultrasound
- mapping
- arterial calcifications
Introduction
Arterial calcifications are common in patients end-stage kidney disease (ESKD) on dialysis. Risk factors for arterial calcification include advanced age, diabetes, smoking, deranged CKD-related mineral and bone disease (MBD), as well as hypertension and hyperlipidemia [1-3]. The presence of arterial calcifications worsens the prognosis of patients with CKD and increases the likelihood of cardiovascular events [4, 5]. Furthermore, arterial calcifications also reduce the possibility of arterio-venous fistula (AVF) formation and maturation and increase the likelihood of complications, especially distal ischemia [6-9]. This is a significant disadvantage, as AVF is the optimal vascular access for patients on maintenance hemodialysis and thus their lifeline.
In a large biopsy study radial artery calcifications were reported in 21% of patients [2], whil a smaller biopsy study reported a 20% prevalence of calcifications [3]. Some large contemporary cohorts on preoperative vascular mapping report similar prevalence rates. Srivastava reported a 9% prevalence of calcifications in the radial artery [13], while our group reported a much higher 20% prevalence in elderly patients [14]. Arterial calcifications represent a significant clinical problem among elderly and diabetic patients and influence the decision of where to place an AVF.
How to assess calcified arteries
It was shown that preoperative ultrasound examination of the arteries often significantly changes the surgical plan, particularly when radio-cephalic AVF is planned [22]. The question that must be answered by clinical and ultrasound examination is: if an adequate vein is available, when should a calcified radial artery be declared too calcified and therefore an AVF creation attempt futile?
B-mode ultrasound
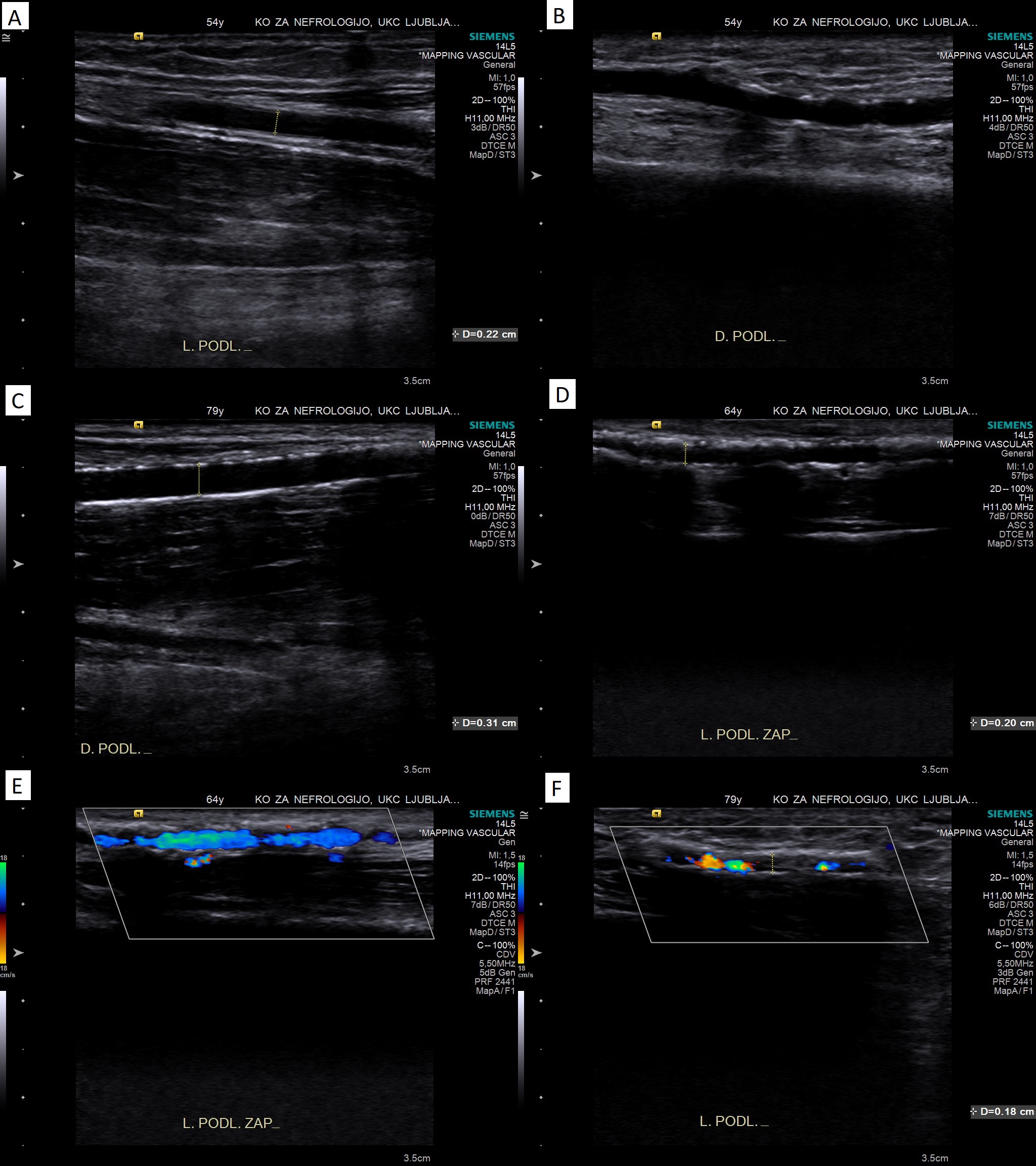
Ultrasound mapping of vessels is considered as standard of care for preoperative evaluation of arteries (and veins) in the developed world. With B-mode ultrasound calcifications of the arterial wall can readily be seen and also quantified. There is no commonly accepted grading scale for quantifying calcifications in upper extremity arteries. Nevertheless, there are several grading scales used in the literature. The intensity of the calcifications might be most important in assessing the suitability of the artery for creation of vascular access, as severe circumferential calcifications make the artery incompressible for clamping, make the creation of the anastomosis much more difficult and outcomes are much worse. Based on existing literature [1, 17, 26, 27] and clinical experience, a semiquantitative calcifications grading scale can be proposed for arteries of the arm (see Table 1 and Figure 1).
Table 1: Proposed grading of severity of arterial calcifications based on B-mode and Color Doppler ultrasound findings; adapted from [1, 17, 26, 27].
|
Calcification grade |
B-mode image |
Color Doppler image |
Appropriateness for fistula creation |
|
none |
smooth vessel wall, |
homogenous signal |
yes |
|
mild |
minor wall structure irregularities, increased echogenicity with spotty calcifications but without distal shadowing |
homogenous signal |
yes |
|
moderate |
irregular wall structure, intermittent calcifications with distal shadowing or linear calcifications with incomplete distal shadowing |
partly homogenous signal (drop-outs <50% of visible artery length) |
likely |
|
severe |
irregular wall structure with diminished separation from surrounding tissue, continuous calcification of the wall with distal shadowing |
very patchy (drop-outs >50% of visible artery length) or almost absent signal |
likely not / careful consideration |
Figure 1: Illustrative B-mode and color Doppler images of the proposed grading of severity of calcifications. Panel A: no calcifications (clear separation of intima and media); panel B: mild calcifications (increased echogenicity/spotty calcifications without distal shadowing); panel C: moderate calcifications (linear calcifications with incomplete distal shadowing); panel D: severe calcifications (diminished separation from surrounding tissue, continuous calcification with distal shadowing); panel E: partly homogenous color Doppler signal in a calcified artery; panel F: very patchy color Doppler signal in a calcified artery.
Compressibility of the artery
Compressibility of a calcified artery can be assessed prior to surgery in cross-sectional view. The ultrasound probe can be pressed at an appropriate angle so that it compresses the artery onto the bone (radius) [27]. Compressibility should be tested at several sites in the area of desired anastomosis, as calcifications are focal. This test mimics clamping the artery during surgery and can likely prevent unnecessary surgery.
Color Doppler assessment
Different color and pulsed-wave Doppler parameters can be used to assess the suitability of the artery for AVF creation (see Table 1 and Figure 2, panel E and F). First, the homogeneity of the color Doppler signal can be assessed. Sonographic drop-out, which are visible as gaps in the color Doppler signal, are caused by dense calcifications that also cause distal shadowing [30, 31]. Homogeneity or continuity of the color Doppler signal within the arterial lumen can therefore be used to assess the severity of observed arterial wall calcifications, when they are present [27].
Outcomes of AVFs placed on calcified arteries
The available literature on radiocephalic AVF placement on calcified radial arteries is summarized in Table 2. We believe that these acceptable results make construction of a radiocephalic AVF on a moderately or even severely calcified radial artery a worthwhile attempt if other conditions (vein and artery diameters) are optimal.
Table 2: Outcomes of radiocephalic AVFs placed on calcified radial arteries.
|
reference |
N in the calcified group |
degree of calcifications |
clinical maturation rate |
1-year secondary patency rate |
|
Sedlacek, 2001 [37] |
25 |
not graded |
80% |
/ |
|
Georgiadis, 2014 [9] |
47 |
moderate? |
89%* |
52% |
|
Srivastava, 2018 [13] |
17 |
not graded |
48% |
/ |
|
Kim, 2019 [51] |
18 |
mild (spoty) |
93% |
/ |
|
Suresh Kumar, 2019 [7] |
9 |
moderate / severe? |
22% |
/ |
|
Sadasivan, 2021 [8] |
11 3 |
mild / moderate severe |
73% 33% |
/ / |
|
Gubensek, 2022 [27] |
18 |
moderate / severe |
67% |
66% |
* calculated as 42 / 47 (2 immediate failures, 3 non-matured AVFs) [9]
Conclusions
Noninvasive ultrasound examination is probably the best tool for morphologic and functional assessment of the arteries. Precise evaluation of the suitability of a calcified artery for possible creation of an AVF remains a challenging task. The decision to perform a forearm AVF instead of an elbow AVF whenever possible, even in the presence of calcifications, is not only an academic or policy issue, because forearm AVFs have much fewer ischemic complications. Therefore, arterial assessment is of utmost importance if we are to create distal radiocephalic AVFs in our elderly patients whenever possible without burdening them with futile surgical attempts. A radial artery should probably be dismissed as too calcified when there are severe calcifications present with very patchy color Doppler and the artery is incompressible with the ultrasound probe. These may be the best predictors that the artery can be clamped and the anastomosis sutured without extreme surgical measures, although further studies are needed.
References
- Lanzer P, Boehm M, Sorribas V, et al. Medial vascular calcification revisited: review and perspectives. Eur Heart J. 2014; 35(23):1515-25. DOI: 10.1093/eurheartj/ehu163.
- Chen Z, Zhou Y, Yang T. Histopathological assessment of radial artery calcification in patients with end-stage kidney disease. Ren Fail. 2021; 43(1):362-70. DOI: 10.1080/0886022X.2021.1889600.
- Wang N, Yang J, Yu X, et al. Radial artery calcification in end-stage renal disease patients is associated with deposition of osteopontin and diminished expression of alpha-smooth muscle actin. Nephrology (Carlton). 2008; 13(5):367-75. DOI: 10.1111/j.1440-1797.2008.00941.x.
- Chen J, Budoff MJ, Reilly MP, et al. Coronary artery calcification and risk of cardiovascular disease and death among patients with chronic kidney disease, JAMA Cardiol. 2017; 2(6):635–43. DOI: 10.1001/jamacardio.2017.0363.
- Erlandsson H, Qureshi AR, Ripsweden J, et al. Scoring of medial arterial calcification predicts cardiovascular events and mortality after kidney transplantation. J Intern Med. 2022; 291:813–23. DOI: 10.1111/joim.13459.
- Schmidli J, Widmer MK, Basile C, et al. Vascular Access: 2018 Clinical Practice Guidelines of the European Society for Vascular Surgery (ESVS). Eur J Vasc Endovasc Surg. 2018; 55: 757e818. DOI: 10.1016/j.ejvs.2018.02.001.
- Suresh Kumar J, Sajeev Kumar KS, Arun Thomas ET, et al. Prediction model for successful radiocephalic arteriovenous fistula creation in patients with diabetic nephropathy. Saudi J Kidney Dis Transpl. 2019; 30(5):1058-64. DOI: 10.4103/1319-2442.270261.
- Sadasivan K, Kunjuraman U, Murali B, et al. Factors Affecting the Patency of Radiocephalic Arteriovenous Fistulas Based on Clinico-Radiological Parameters. Cureus. 2021; 13(3):e13678. DOI: 10.7759/cureus.13678.
- Georgiadis GS, Georgakarakos EI, Antoniou GA, et al. Correlation of pre-existing radial artery macrocalcifications with late patency of primary radiocephalic fistulas in diabetic hemodialysis patients. J Vasc Surg. 2014; 60(2):462-70. DOI: 10.1016/j.jvs.2014.02.042.
- Allon M, Robbin ML, Umphrey HR, et al. Preoperative arterial microcalcification and clinical outcomes of arteriovenous fistulas for hemodialysis. Am J Kidney Dis. 2015; 66: 84–90. DOI: 10.1053/j.ajkd.2014.12.015.
- El Khoury R, Russeau AP, Patel N, et al. Reliability of preoperative venous mapping ultrasonography in predicting for autogenous arteriovenous fistula maturation. J Vasc Surg. 2021; 73(5):1787-1793. DOI: 10.1016/j.jvs.2020.09.035.
- Li HL, Chan YC, Cui D, et al. Predictors of Primary Functional Maturation of Autogenous Radiocephalic Arteriovenous Fistula in a Cohort of Asian Patients. Ann Vasc Surg. 2020; 66:326-33. DOI: 10.1016/j.avsg.2019.12.029.
- Srivastava A, Sureka SK, Prabhakaran S, et al. Role of Preoperative Duplex Ultrasonography to Predict Functional Maturation of Wrist Radiocephalic Arteriovenous Fistula: A Study on Indian Population. Indian J Nephrol. 2018; 28(1):10-14. DOI: 10.4103/ijn.IJN_134_16.
- Persic V, Ponikvar R, Buturovic-Ponikvar J. Preoperative ultrasonographic mapping of blood vessels before arteriovenous fistula construction in elderly patients with end-stage renal disease. Ther Apher Dial. 2009; 13(4):334-9. DOI: 10.1111/j.1744-9987.2009.00735.x.
- Cho M, Kim JS, Cho S, et al. Baseline characteristics of arm vessels by preoperative duplex ultrasonography in Korean patients for hemodialysis vascular access. J Vasc Access. 2019; 20(6):646-651. DOI: 10.1177/1129729819838168.
- Lok CE, Huber TS, Lee T, et al. KDOQI Clinical Practice Guideline for Vascular Access: 2019 Update. Am J Kidney Dis. 2020; 75(4 Suppl 2):S1-S164. DOI: 10.1053/j.ajkd.2019.12.001.
- Malovrh M. Native arteriovenous fistula: preoperative evaluation. Am J Kidney Dis. 2002; 39(6):1218-25. DOI: 10.1053/ajkd.2002.33394.
- Ferring M, Claridge M, Smith SA, et al. Routine preoperative vascular ultrasound improves patency and use of arteriovenous fistulas for hemodialysis: a randomized trial. Clin J Am Soc Nephrol. 2010; 5(12):2236-44. DOI: 10.2215/CJN.02820310.
- Mihmanli I, Besirli K, Kurugoglu S, et al. Cephalic vein and hemodialysis fistula: surgeon’s observation versus color Doppler ultrasonographic findings. J Ultrasound Med 2001; 20:217e22. DOI: 10.7863/jum.2001.20.3.217
- Alves Lopes JR, de Barros Marques AL, Correa JA. Randomised clinical study of the impact of routine preoperative Doppler ultrasound for the outcome of autologous arteriovenous fistulas for haemodialysis. J Vasc Access. 2021; 22(1):107-14. DOI: 10.1177/1129729820927273.
- Gallieni M, Hollenbeck M, Inston N, et al. Clinical practice guideline on peri- and postoperative care of arteriovenous fistulas and grafts for haemodialysis in adults. Nephrol Dial Transplant. 2019; 34: ii1–ii42. DOI: 10.1093/ndt/gfz072
- Kim JJ, Koopmann M, Ihenachor E, et al. The addition of ultrasound arterial examination to upper extremity vein mapping before hemodialysis access. Ann Vasc Surg 2016; 33: 109–15. DOI: 10.1016/j.avsg.2016.02.001.
- American Institute of Ultrasound in Medicine. AIUM practice guideline for the performance of ultrasound vascular mapping for preoperative planning of dialysis access. J Ultrasound Med. 2012; 31(1):173-81. DOI: 10.7863/jum.2012.31.1.173.
- Liu KH, Chu WCW, Kong APS, et al. US assessment of medial arterial calcification: a sensitive marker of diabetes-related micro vascular and macrovascular complications. Radiology. 2012; 265(1):294-302. DOI: 10.1148/radiol.12112440.
- Tian J, Tang G, Xu X, et al. Different Ultrasound Scoring Methods for Assessing Medial Arterial Calcification: Association with Diabetic Complications. Ultrasound Med Biol. 2020; 46(6):1365-72. DOI: 10.1016/j.ultrasmedbio.2020.01.024.
- Taylor C, Zielinski LP, Chowdhury MM, et al. Defining the Role of Duplex Ultrasound Assessment to Determine Severity of Arterial Calcification: An Analysis of the Superficial Femoral Artery. Journal for Vascular Ultrasound. 2020; 44(2):74-8. DOI:10.1177/1544316720910550.
- Gubensek J. Doppler ultrasound assessment of calcified radial arteries prior to radio-cephalic arterio-venous fistula placement - an observational study. J Vasc Access. 2023, in press. DOI: 10.1177/11297298221143598.
- Georgakarakos E, Kostoglou P. The "No Clamp" Technique for Anastomosis in Calcified Vessels. Eur J Vasc Endovasc Surg. 2020; 59(3):483. DOI: 10.1016/j.ejvs.2019.11.036.
- Napoli M, Barbarini S, Ria P, et al. The intraoperative intravascular lithotripsy to recruit a calcified radial artery for creating a distal radio-cephalic fistula. J Vasc Access. 2023; 24(2):300-4. DOI: 10.1177/11297298211017029.
- Rocha-Singh KJ, Zeller T, Jaff MR. Peripheral arterial calcification: prevalence, mechanism, detection, and clinical implications. Catheter Cardiovasc Interv. 2014; 83(6):E212-20. DOI: 10.1002/ccd.25387.
- Pajek J, Malovrh M. Preoperative ultrasound still valuable for radio-cephalic arteriovenous fistula creation? J Vasc Access. 2017; 18(Suppl. 1):5-9. DOI: 10.5301/jva.5000672.
- Vallespin J, Meola M, Ibeas J. Upper limb anatomy and preoperative mapping. J Vasc Access. 2021; 22(1_suppl):9-17. DOI: 10.1177/11297298211046827.
- Horst VD, Nelson PR, Mallios A, et al. Avoiding hemodialysis access-induced distal ischemia. J Vasc Access. 2021; 22(5):786-94. DOI: 10.1177/1129729820943464
- Zachaus M, Herrmann V, Plehn A et al. Sonographic findings in the diagnostic course of vascular access for hemodialysis. Med Klin (Munich) 2005; 100: 1–5. DOI: 10.1007/s00063-005-1112-3.
- Bardelli M, Veglio F, Arosio E, et al. New intrarenal echo-Doppler velocimetric indices for the diagnosis of renal artery stenosis. Kidney Int. 2006; 69(3):580-7. DOI: 10.1038/sj.ki.5000112.
- Trihan JE, Mahe G, Laroche JP, et al. Arterial Blood-Flow Acceleration Time on Doppler Ultrasound Waveforms: What Are We Talking About? J Clin Med. 2023; 12(3):1097. DOI: 10.3390/jcm12031097.
- Sedlacek M, Teodorescu V, Falk A, Vassalotti JA, Uribarri J. Hemodialysis access placement with preoperative noninvasive vascular mapping: comparison between patients with and without diabetes. Am J Kidney Dis. 2001; 38(3):560-4. DOI: 10.1053/ajkd.2001.26873.
- Lockhart ME, Robbin ML, Allon M. Preoperative sonographic radial artery evaluation and correlation with subsequent radiocephalic fistula outcome. J Ultrasound Med. 2004; 23(2):161-8. DOI: 10.7863/jum.2004.23.2.161.
- Smith GE, Gohil R, Chetter IC. Factors affecting the patency of arteriovenous fistulas for dialysis access. J Vasc Surg. 2012; 55(3):849-55. DOI: 10.1016/j.jvs.2011.07.095.
- Kim ES, Sharma AM, Scissons R, et al. Interpretation of peripheral arterial and venous Doppler waveforms: A consensus statement from the Society for Vascular Medicine and Society for Vascular Ultrasound. Vasc Med. 2020; 25(5):484-506. DOI: 10.1177/1358863X20937665.
- Spronk S, den Hoed PT, de Jonge LCW, et al. Value of the duplex waveform at the common femoral artery for diagnosing obstructive aortoiliac disease. J Vasc Surg. 2005; 42(2):236-42. DOI: 10.1016/j.jvs.2005.04.048.
- Brouwers JJWM, Willems SA, Goncalves LN, et al. Reliability of bedside tests for diagnosing peripheral arterial disease in patients prone to medial arterial calcification: A systematic review. ClinicalMedicine. 2022; 50:101532. DOI: 10.1016/j.eclinm.2022.101532.
- Trihan JE, Mahe G, Croquette M, et al. Acceleration Time of Distal Arteries to Diagnose Severe Peripheral Arterial Disease. Front. Cardiovasc. Med. 2022. 8:744354. DOI: 10.3389/fcvm.2021.744354.
- Yagyu T, Funabashi S, Yoneda S, et al. Novel Evaluation Method for Lower Extremity Peripheral Artery Disease With Duplex Ultrasound - Usefulness of Acceleration Time. Circ J. 2020; 84(11):1990-8. DOI: 10.1253/circj.CJ-20-0427.
- Rosenberry R, Nelson XMD. Reactive hyperemia: a review of methods, mechanisms, and considerations. Am J Physiol Regul Integr Comp Physiol. 2020; 318: R605–18. DOI: 10.1152/ajpregu.00339.2019.
- Wall LP, Gasparis A, Callahan S, et al. Impaired hyperemic response is predictive of early access failure. Ann Vasc Surg. 2004; 18: 167–171. DOI 10.1007/s10016-004-0006-9.
- Kanta Ghosh N, Kumar Bhattacharjee H, Prajapati O, et al. Impact of clinical parameters and vascular haemodynamics on arterio-venous fistula maturation in patients with end stage renal disease: A prospective study on Indian patients. J Vasc Access. 2022; 23(4):508-14. DOI: 10.1177/11297298211001158.
- Meena P, Bhargava V, Sehrawat S, et al. Stretching the boundaries: suitability of an arteriovenous fistula in elderly patients on hemodialysis-a northern India experience. Int Urol Nephrol. 2022; 54(3):671-8. DOI: 10.1007/s11255-021-02941-4.
- Masengu A, McDaid J, Maxwell AP, Hanko JB. Preoperative radial artery volume flow is predictive of arteriovenous fistula outcomes. J Vasc Surg. 2016; 63(2):429-35. DOI: 10.1016/j.jvs.2015.08.106.
- Bonucchi D, Cappelli G, Albertazzi A. Which is the preferred vascular access in diabetic patients? A view from Europe. Nephrol Dial Transplant. 2002; 17(1):20-2. DOI: 10.1093/ndt/17.1.20.
- Kim SM, Jung IM, Kim D, et al. Effect of Inflow Arterial Calcification on Arteriovenous Fistula Maturation. Ann Vasc Surg. 2019; 58:331-337. DOI: 10.1016/j.avsg.2018.10.057.
- Huber TS, Larive B, Imrey PB, et al. Access-related hand ischemia and the Hemodialysis Fistula Maturation Study. J Vasc Surg. 2016; 64(4):1050-8.e1. DOI: 10.1016/j.jvs.2016.03.449.
- Kudlaty EA, Kendrick DE, Allemang MT, et al. Upper Extremity Steal Syndrome Is Associated with Atherosclerotic Burden and Access Configuration. Ann Vasc Surg. 2016; 35:82-7. DOI: 10.1016/j.avsg.2016.01.058.
- Morsy AH, Kulbaski M, Chen C, et al. Incidence and characteristics of patients with hand ischemia after a hemodialysis access procedure. J Surg Res. 1998; 74(1):8-10. DOI: 10.1006/jsre.1997.5206.
- Vajdic Trampuz B, Arnol M, Gubensek J, et al. A national cohort study on hemodialysis arteriovenous fistulas after kidney transplantation - long-term patency, use and complications. BMC Nephrol. 2021; 22:344. DOI: 10.1186/s12882-021-02550-4.
- Beathard GA, Spergel LM. Hand ischemia associated with dialysis vascular access: an individualized access flow-based approach to therapy. Semin Dial. 2013; 26(3):287-314. DOI: 10.1111/sdi.12088.
- Jennings WC, Mallios A, Mushtaq N. Proximal radial artery arteriovenous fistula for hemodialysis vascular access. J Vasc Surg. 2018; 67(1):244-253. DOI: 10.1016/j.jvs.2017.06.114.
- van Hoek F, Scheltinga MR, Kouwenberg I, et al. Steal in hemodialysis patients depends on type of vascular access. Eur J Vasc Endovasc Surg. 2006; 32(6):710-7. DOI: 10.1016/j.ejvs.2006.05.018.
- Tordoir JHM, Bode AS, van Loon MM. Preferred strategy for hemodialysis access creation in elderly patients. Eur J Vasc Endovasc Surg. 2015; 49(6):738-743. DOI: 10.1016/j.ejvs.2015.02.006.
- Lazarides MK, Staramos DN, Kopadis G, et al. Onset of arterial 'steal' following proximal angioaccess: immediate and delayed types. Nephrol Dial Transplant. 2003; 18(11):2387-90. DOI: 10.1093/ndt/gfg346.
- Keuter XHA, Kessels AGH, de Haan MH, et al. Prospective evaluation of ischemia in brachial-basilic and forearm prosthetic arteriovenous fistulas for hemodialysis. Eur J Vasc Endovasc Surg. 2008; 35(5):619-24. DOI: 10.1016/j.ejvs.2007.11.004.
- Ferraresi R, Acuna-Valerio J, Ferraris M, et al. Angiographic study of upper limb vascularization in a large cohort of hemodialysis patients with critical hand ischemia. Minerva Cardioangiol. 2016; 64(6):642-7.
- Tatoulis J, Royse AG, Buxton BF, et al. The radial artery in coronary surgery: 5-year experience-clinical and angiographic results. Ann Thorac Surg 2002; 73:143-8. DOI: 10.1016/s0003-4975(01)03290-8.
- Manabe S, Tabuchi N, Tanaka H, et al. Hand circulation after radial artery harvest for coronary artery bypass grafting. J Med Dent Sci. 2005; 52: 101–37.
- Abu-Omar Y, Mussa S, Anastasiadis K, et al. Duplex Ultrasonography Predicts Safety of Radial Artery Harvest in the Presence of an Abnormal Allen Test. Ann Thorac Surg 2004; 77:116-9. DOI:10.1016/S0003-4975(03)01515-7.
- van der Heijden DJ, van Leeuwen MAH, Ritt MJPF, van de Ven PM, van Royen N. Chronic radial artery occlusion does not cause exercise induced hand ischemia. J Interv Cardiol. 2018; 31(6):949-56.
- Khalil IM, Livingston DH. The management of steal syndrome occurring after access for dialysis. J Vasc Surg. 1988; 7(4):572-3. DOI: 10.1067/mva.1988.avs0070572.
- Valentine RJ, Bouch CW, Scott DJ, et al. Do preoperative finger pressures predict early arterial steal in hemodialysis access patients? A prospective analysis. J Vasc Surg. 2002; 36(2):351-6. DOI: 10.1067/mva.2002.125848.
- Beathard GA, Jennings WC, Wasse H, et al. ASDIN white paper: Assessment and management of hemodialysis access-induced distal ischemia by interventional nephrologists. J Vasc Access. 2020; 21(5):543-53. DOI: 10.1177/1129729819894774.
- Yadav R, Gerrickens MWM, Teijink JAW, Scheltinga MRM. Systolic finger pressures during an Allen test before hemodialysis access construction predict severe postoperative hand ischemia. J Vasc Surg. 2021; 74(6):2040-2046. DOI: 10.1016/j.jvs.2021.07.127.
This entry is adapted from the peer-reviewed paper 10.3390/diagnostics13162660
