Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Immunology
Long noncoding RNAs (lncRNAs) are molecules >200 bases in length without protein-coding functions implicated in signal transduction and gene expression regulation via interaction with proteins or RNAs, exhibiting various functions. The expression of lncRNAs has been detected in many cell types, including macrophages, a type of immune cell involved in acute and chronic inflammation, removal of dead or damaged cells, and tissue repair. Increasing evidence indicates that lncRNAs play essential roles in macrophage functions and disease development.
- atherosclerosis
- inflammation
- lncRNA
- macrophage
- sepsis
1. Introduction
Recent advances in molecular biology have revealed that although noncoding RNAs (ncRNAs) are not translated into proteins, they play various roles in cellular processes and disease pathogeneses. Long ncRNAs (lncRNAs) are >200 nucleotides in length and have been extensively researched in various fields of biology [1]. Nuclear lncRNAs have been implicated in regulating chromatin organization, gene transcription, RNA splicing, and epigenetic modifications [2,3,4,5]. Certain lncRNAs that exhibit structural features similar to those of mRNAs can be transported to the cytoplasm to modulate signaling pathways and post-transcriptional gene expression regulation by affecting mRNA stability and translation or sponging microRNAs (miRNAs) to block their function [6,7,8,9,10].
Macrophages exhibit immunoregulatory functions during acute and chronic inflammation, pathogenesis of various diseases, and cancer development. They are categorized into M1 and M2 functional groups. M1 macrophages (also known as “killer” or classically activated macrophages) phagocytose pathogens and foreign substances and promote inflammation, whereas M2 macrophages (also known as “repair” or alternatively activated macrophages) mediate tissue repair and inflammation resolution [11,12]. M1 macrophages metabolize arginine to nitric oxide and synthesize ATP via glycolysis. Furthermore, the mitochondrial citric acid cycle is shut down in these cells. Conversely, M2 macrophages metabolize arginine into ornithine or proline and mostly synthesize ATPs via the citric acid cycle [13]. The bacterial endotoxin lipopolysaccharide (LPS) and interferon (IFN)-γ induce the differentiation of undifferentiated (M0) macrophages into M1 macrophages, which produce a range of proinflammatory cytokines, including tumor necrosis factor (TNF)-α, interleukin (IL)-1, and IL-12. These cytokines and M1 macrophages have been associated with acute inflammation and tissue damage. Conversely, M2 differentiation, induced by IL-4 and IL-13, is characterized by the production of anti-inflammatory cytokines, such as IL-10 and transforming growth factor (TGF)-β. These cytokines and M2 macrophages help regulate immune responses and promote tissue repair [13,14]. Given that the activation and differentiation pathways and immunological roles of M1 and M2 macrophages differ, a detailed understanding of the factors that maintain or regulate the balance between them is important to effectively treat various diseases, such as autoimmune diseases, inflammatory bowel diseases, diabetes, obesity, rheumatoid arthritis (RA), and systemic sclerosis [13,15].
2. Atherosclerosis
Atherosclerosis is a chronic inflammatory disease characterized by the narrowing and hardening of arteries due to the accumulation of lipid-laden plaques on their inner walls. Macrophages engulf modified low-density lipoprotein (LDL) particles, such as oxidized LDL (oxLDL), and differentiate into foam cells, thereby aggravating chronic inflammatory conditions, stimulating plaque growth, and destabilizing plaques [18,19]. Atherosclerosis has been associated with various health problems, including coronary artery diseases (CADs) and stroke. M1 and M2 macrophages have been implicated in atherogenesis. M1 macrophages promote inflammation and plaque rupture by producing proinflammatory cytokines, chemokines, reactive oxygen species (ROS), and extracellular matrix-degrading enzymes. Conversely, M2 macrophages resolve inflammation by releasing anti-inflammatory cytokines, such as IL-10 [13,20,21].
2.1. LncRNAs That Promote Inflammation and Foam Cell Formation
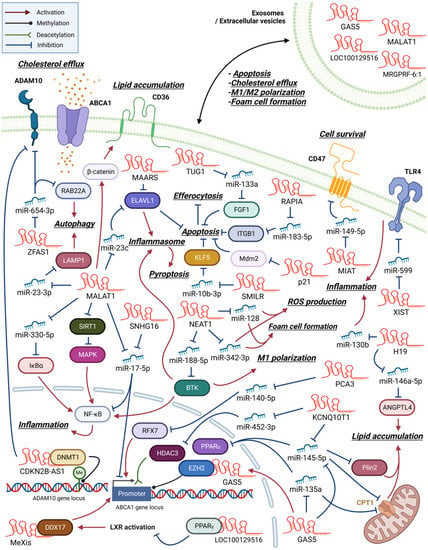
Many macrophage lncRNAs involved in atherosclerosis development are induced by oxLDL and promote foam cell formation through enhancing inflammatory changes (Figure 2). Previous studies found that lncRNA H19 expression was upregulated after oxLDL treatment in peripheral blood mononuclear cells of patients with CAD, plaque macrophages of an atherosclerotic mouse model, and RAW264.7 murine macrophage-like cells [22,23]. Transfecting H19-specific short hairpin RNA (shRNA) into RAW264.7 cells decreased oxLDL-induced lipid accumulation and proinflammatory mediator expression by regulating miR-130b activity [24].

Figure 2. An overview of macrophage lncRNAs involved in atherosclerosis development. LncRNAs exert their functions through both direct and indirect mechanisms. Certain lncRNAs act as miRNA sponges, indirectly regulating protein expression by inhibiting miRNA function. In contrast, other lncRNAs directly interact with proteins to modulate their activity. Additionally, some lncRNAs can regulate gene expression through epigenetic modifications. Within the figures, arrows are employed to indicate the molecule responsible for activating or inhibiting its specific target.
NEAT1 (LINC00084, shortened from either nuclear paraspeckle assembly transcript 1 or nuclear enriched abundant transcript 1) is a nucleus-restricted lncRNA involved in the formation of paraspeckles, which are subnuclear structures implicated in antiviral responses [26]. NEAT1 expression is upregulated in oxLDL-treated THP-1 cells, a human monocytic leukemia cell line with macrophage-like properties. Furthermore, NEAT1 participates in the formation of paraspeckles and the development of subsequent proinflammatory responses by regulating p65 phosphorylation. Additionally, NEAT1 modulates lipid uptake by regulating the expression of a scavenger receptor, CD36 [27]. The treatment of RAW264.7 cells with oxLDL increases NEAT1 expression, which in turn stimulates proinflammatory cytokine and ROS production, subsequently promoting foam cell formation by sponging miR-128 [28].
In THP-1 cells, oxLDL treatment upregulated lncRNA urothelial cancer-associated 1 (UCA1) expression levels, which further exacerbated atherosclerotic events, such as CD36 expression, foam cell formation, and ROS generation via sponging miR-206 [32]. In atherosclerotic animal models, lncRNA dynamin 3 opposite strand (Dnm3os) expression was increased in atherosclerotic plaques. Dnm3os regulated macrophage proinflammatory activities via the miR-27b-3p/signaling lymphocytic activation molecule 7 (SLAMF7) axis [33]. SLAMF7 is a membrane protein whose expression is upregulated in macrophages during phagocytosis and macrophage differentiation in atherosclerotic plaques [34].
2.2. LncRNAs That Regulate Cholesterol Efflux and Foam Cell Formation
Numerous clinical and animal studies have demonstrated that defects in reverse cholesterol transport and cholesterol efflux are associated with an increased risk of cardiovascular diseases and atherosclerosis [18,19]. ATP-binding cassette subfamily A member 1 (ABCA1)-mediated cholesterol efflux reduces the formation of lipid-laden foam cells in atherosclerotic plaques. Because regulation of cholesterol efflux is crucial for the prevention of atherosclerosis, ABCA1 has been one of the primary targets in lncRNA research.
The lncRNA macrophage-expressed liver X receptor (LXR)-induced sequence (MeXis) is reportedly involved in LXR-dependent transcriptional activation of Abca1 by guiding the promoter binding of the transcription coactivator DEAD-box helicase 17 (DDX17). Furthermore, bone marrow cells from MeXis-deficient mice exhibited altered chromosome architecture at the Abca1 locus, impaired cholesterol efflux, and accelerated atherosclerosis development. Notably, the genes encoding ABCA1 and MeXis are located near one another to ensure tissue-selective activation of this regulatory circuit [35].
In contrast, the lncRNA growth arrest-specific 5 (GAS5) exerts inhibitory effects on ABCA1 function through its interaction with and stabilization of the enhancer of zeste homolog 2 (EZH2), a chromatin-repressive complex known to promote trimethylation of lysine 27 (H3K27) at the Abca1 promoter [38]. Notably, a significant elevation in GAS5 levels was detected in the serum of patients with coronary heart disease, exhibiting a correlation with heightened proinflammatory markers [39].
2.3. LncRNAs That Regulate Macrophage Apoptosis, Pyroptosis, or Autophagy in Atherosclerosis
Cellular processes such as apoptosis, pyroptosis, and autophagy need to be balanced with macrophage proliferation. Disruption of the balance may destabilize atherosclerotic plaques. Apoptotic cell death, especially in overstimulated or exhausted foam cells, can enhance inflammation and trigger blood clot formation, which may lead to heart attack or stroke [18,19].
LncRNAs that inhibit apoptosis tend to aggravate atherogenesis [53,60,61,62,63]. By suppressing apoptosis, macrophages are allowed to proliferate and promote plaque formation. In addition to inhibiting apoptosis, lncRNAs such as taurine-upregulated gene 1 (TUG1) and X-inactive specific transcript (XIST) enhance inflammation via fibroblast growth factor 1 and Toll-like receptor 4 (TLR4), respectively [60,61]. LncRNAs associated with the progression and intervention of atherosclerosis (RAPIA) and smooth-muscle-induced lncRNA (SMILR) also inhibit apoptosis and enhance atherosclerosis by regulating cellular receptor integrin beta 1 (ITGB1) and a transcription factor, Krueppel-like factor 5 (KLF5), respectively [62,63].
The progression of atherosclerosis may also be aggravated by defects in a process known as efferocytosis, the clearance of apoptotic cells by macrophages. Simion et al., detected high-level expression of a macrophage-associated atherosclerotic lncRNA sequence (MAARS) in the aortic intima of atherogenic animal models, and it decreased with the regression of atherosclerosis. Knockdown experiments indicated that MAARS promotes macrophage apoptosis, thereby inhibiting efferocytosis, through its interaction with HuR (ELAVL1), an RNA-binding protein with an apoptosis regulator function [57]. Another lncRNA, MI-associated transcript (MIAT), directly affects efferocytosis [55]. Its expression has been detected in the serum of patients with advanced atherosclerosis and necrotic core macrophages.
2.4. LncRNAs Functioning via Exosomes in Atherogenesis
Many studies have indicated that exosomes can be used as carriers of lncRNA to regulate cellular activities of neighboring cells. The upregulation of lnc-MRGPRF-6:1 expression and as its correlation with levels of proinflammatory mediators have been detected in the plasma exosomes of patients with CAD [67]. The expression level of lnc-MRGPRF-6:1 following M1 induction was higher than that following M2 induction in THP-1 cells. The knockout of lnc-MRGPRF-6:1 reduced ROS generation, lipid accumulation, and subsequent foam cell formation. Furthermore, lnc-MRGPRF-6:1 knockout in human monocyte-derived macrophages suppressed M1 marker and inflammatory cytokine expression and enhanced M2 marker expression by modulating the TLR4/myeloid differentiation primary response 88 (MyD88)/mitogen-activated protein kinase (MAPK) signaling pathway [67].
2.5. Multiple Function of MALAT1 in Atherogenesis
The lncRNA metastasis-associated lung adenocarcinoma transcript 1 (MALAT1) affects multiple atherosclerotic processes, such as foam cell formation and macrophage apoptosis, autophagy, and pyroptosis (Figure 2). Treating THP-1 cells with oxLDL upregulated MALAT1 expression in a nuclear factor kappa-light-chain-enhancer of activated B cell (NF-κB)-dependent manner [43,45]. MALAT1 enhanced lipid uptake by inducing CD36 expression by recruiting β-catenin to its binding sites on the CD36 promoter [45]. MALAT1 also enhanced NF-κB activation and, subsequently, foam cell formation, apoptosis, and inflammation via sponging miR-330-5p [43].
However, there are reports of an opposite role played by MALAT1 in atherosclerosis. For instance, in an apolipoprotein E (apoE)-knockout mouse model, MALAT1 deficiency accelerated inflammation and atherosclerosis. Treating MALAT1-deficient BMDMs with LPS enhanced TNF-α and inducible nitric oxide synthase expression, suppressed matrix metalloproteinase-9 expression, and impaired phagocytic activity [44].
3. Sepsis
Blood monocytes/macrophages and endothelial cells lining the blood vessels respond to gram-negative bacteria infiltration by releasing a flood of chemicals, including cytokines, into circulation to fight the infection. Macrophages can remove pathogens by phagocytosis and regulate the extent of sepsis by producing anti-inflammatory cytokines. However, the production of excess inflammatory cytokines, such as IL-6, IL-1β, and especially TNF-α, may damage the surrounding normal tissues and organs, which can be life-threatening [70,71]. As observed in other diseases, the proinflammatory activity of M1 macrophages aggravates sepsis, whereas the anti-inflammatory activity of M2 macrophages mitigates it [72].
3.1. NEAT1 Enhances Sepsis Progression through Promoting Inflammation
Previous studies have found a considerable increase in NEAT1 levels in serum of patients with sepsis and septic mouse models [73,74,75,76]. These studies agree that NEAT1 is involved in the inflammatory activation of macrophages; however, the targets of its action differ. In THP-1 cells, LPS-induced NEAT1 expression enhances inflammatory responses by modulating the miR-17-5p/TLR4 axis [76]. LPS-stimulated Kupffer or RAW264.7 cells exhibit the expression of NEAT1, which exerts its proinflammatory activities through the Let-7q/TLR4 axis [73]. Other studies have reported that NEAT1 promotes inflammation in LPS-treated RAW264.7 cells by modulating the miR-495-3p/signal transducer and activator of transcription 3 (STAT3), miR-211/phosphoinositide 3-kinase (PI3K)/protein kinase B (AKT), miR-370-3p/thrombospondin-1, or miR-31-5p/POU domain, class 2, transcription factor 1 (POU2F1) axes [74,75,77].
3.2. MALAT1 Promotes M1 Polarization and Inflammation in Sepsis
An increase in lncRNA MALAT1 levels was detected in the serum of late-onset sepsis patients and in activated primary macrophages and macrophage cell lines [79]. MALAT1-knockout mice exhibited reduced inflammation and death upon sepsis induction. Particularly, suppressing MALAT1 expression increased the antioxidant capacity of macrophages through the methyltransferase 16 (METTL16)/methionine adenosyltransferase 2 A (MAT2A) axis, wherein MALAT1 binds to METTL16, thereby stabilizing the METTL16 N6-methyladenosine (m6A) modification activity [79]. MAT2A regulates cellular metabolism and catalyzes S-adenosylmethionine production [80]. Intraperitoneal LPS injection in mice induces septic lung injury, substantially increasing MALAT1 expression in lung tissues. Additional intravenous MALAT1-specific small interfering RNA (siRNA) injection reduces the number of inflammatory cells and cytokine levels in the bronchoalveolar lavage fluid (BALF) of these animal models by inhibiting the p38 MAPK/p65 NF-κB signaling pathway [81]
However, a few studies have reported different observations regarding the role of MALAT1. For instance, Yang et al., reported a significant decrease in MALAT1 serum levels and an increase in hsa-miR-346 levels in patients with sepsis. Activated RAW264.7 cells also exhibit reduced expression of MALAT1. Additional experiments demonstrated that MALAT1 regulates macrophage proliferation through the hsa-miR-346/small mothers against decapentaplegic homolog 3 (SMAD3) axis [83]. SMAD3 is a receptor-regulated signaling adaptor activated by serine kinases.
3.3. Other lncRNAs Involved in Sepsis Development
LPS-induced NF-κB activation in THP-1 cells and the subsequent release of proinflammatory cytokines were shown to be regulated by lncRNA colorectal neoplasia differentially expressed (CRNDE) via the miR-181-5p/TLR4 axis. A considerable increase in CRNDE expression levels and decrease in miR-181-5p expression levels have been detected in the peripheral blood of patients with sepsis.
This entry is adapted from the peer-reviewed paper 10.3390/biomedicines11071905
This entry is offline, you can click here to edit this entry!
