Type 2 diabetes mellitus (T2DM) represents a common metabolic disorder that is characterized by chronic hyperglycemia. For more than half a century, the link between insulin resistance and T2DM has been well recognized. Insulin resistance is not only the most powerful predictor of future development of T2DM, but it is also a therapeutic target. On the other hand, gestational diabetes mellitus (GDM) is one of the most common metabolic disorders of pregnancy and its incidence has considerably increased by 10–100% in the last 20 years. There is ample scientific evidence to suggest a link between the fatty acid-binding protein 4 (FABP4) and insulin resistance, gestational (GDM), and type 2 (T2DM) diabetes mellitus.
- fatty acid-binding protein 4
- insulin resistance
- type 2 diabetes mellitus
1. Introduction
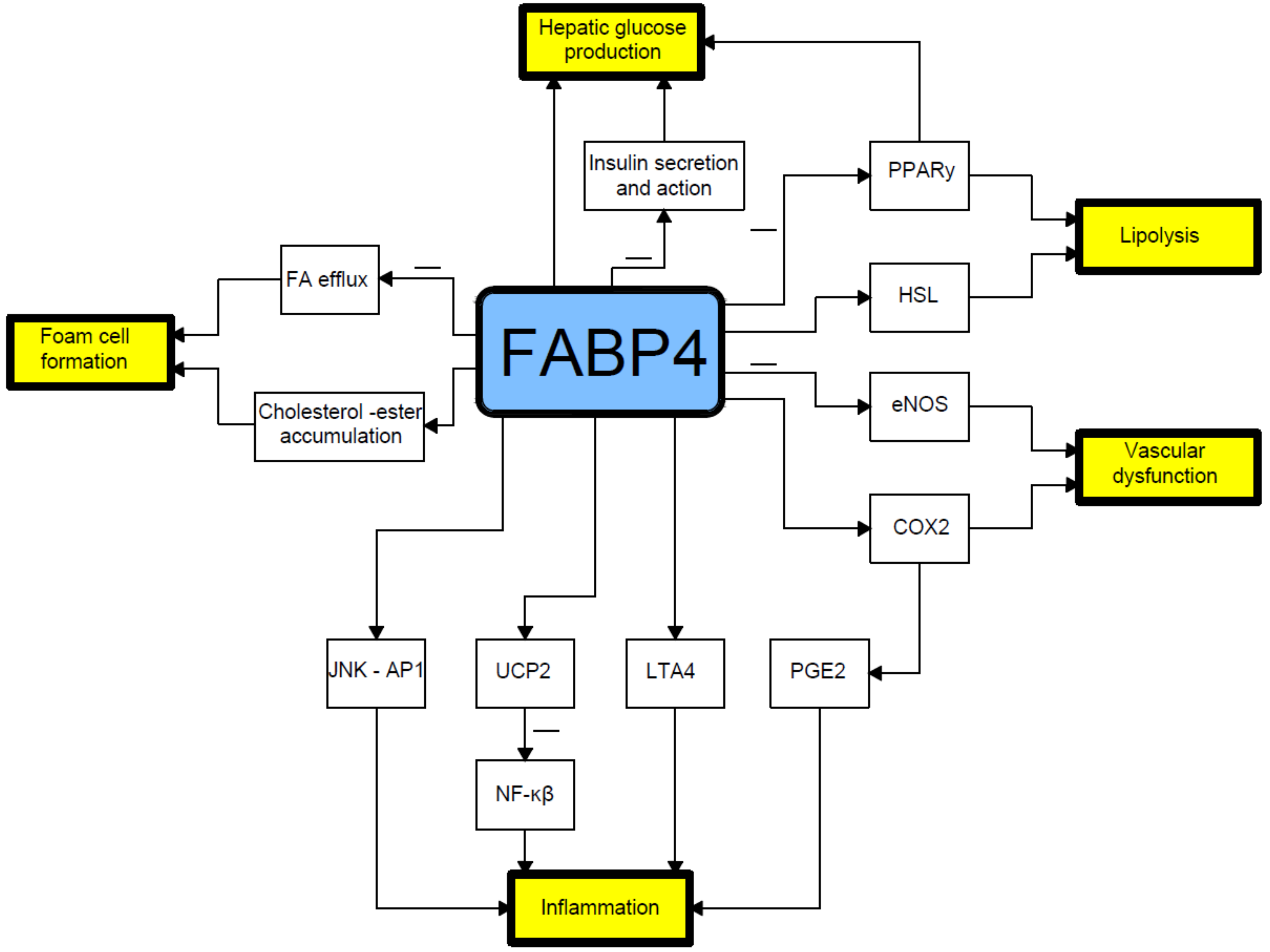
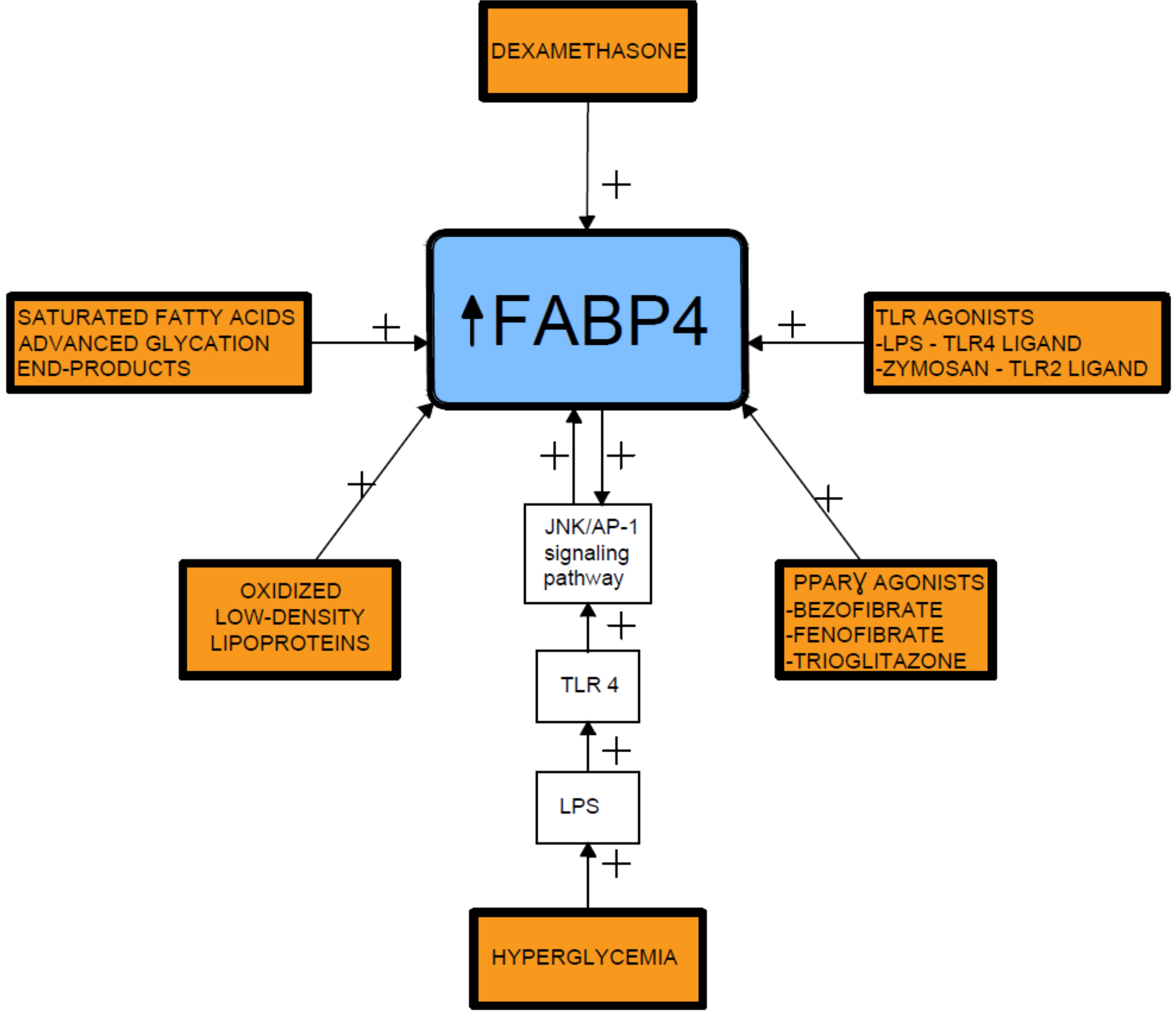
2. Relationship between Fatty Acid-Binding Protein 4 (FABP4) and Insulin Resistance
3. Relationship between Fatty Acid-Binding Protein 4 and Diabetic Nephropathy
4. Relationship between Fatty Acid-Binding Protein 4 and Non-Alcoholic Fatty Liver Disease
This entry is adapted from the peer-reviewed paper 10.3390/cells8030227
References
- Martínez-Micaelo, N.; Rodríguez-Calvo, R.; Guaita-Esteruelas, S.; Heras, M.; Girona, J.; Masana, L. Extracellular FABP4 uptake by endothelial cells is dependent on cytokeratin 1 expression. Biochim. Biophys. Acta Mol. Cell. Biol. Lipids 2018, 1864, 234–244.
- Chen, R.A.; Sun, X.M.; Yan, C.Y.; Liu, L.; Hao, M.W.; Liu, Q.; Jiao, X.Y.; Liang, Y.M. Hyperglycemia-induced PATZ1 negatively modulates endothelial vasculogenesis via repression of FABP4 signaling. Biochem. Biophys. Res. Commun. 2016, 477, 548–555.
- Li, L.; Lee, S.J.; Kook, S.Y.; Ahn, T.G.; Lee, J.Y.; Hwang, J.Y. Serum from pregnant women with gestational diabetes mellitus increases the expression of FABP4 mRNA in primary subcutaneous human pre-adipocytes. Obstet. Gynecol. Sci. 2017, 60, 274–282.
- Harjes, U.; Bridges, E.; McIntyre, A.; Fielding, B.A.; Harris, A.L. Fatty acid-binding protein 4, a point of convergence for angiogenic and metabolic signaling pathways in endothelial cells. J. Biol. Chem. 2014, 289, 23168–23176.
- Cabré, A.; Lázaro, I.; Girona, J.; Manzanares, J.M.; Marimón, F.; Plana, N.; Heras, M.; Masana, L. Fatty acid binding protein 4 is increased in metabolic syndrome and with thiazolidinedione treatment in diabetic patients. Atherosclerosis 2007, 195, e150–e158.
- Garin-Shkolnik, T.; Rudich, A.; Hotamisligil, G.S.; Rubinstein, M. FABP4 attenuates PPARγ and adipogenesis and is inversely correlated with PPARγ in adipose tissues. Diabetes 2014, 63, 900–911.
- Song, J.; Ren, P.; Zhang, L.; Wang, X.L.; Chen, L.; Shen, Y.H. Metformin reduces lipid accumulation in macrophages by inhibiting FOXO1-mediated transcription of fatty acid-binding protein 4. Biochem. Biophys. Res. Commun. 2010, 393, 89–94.
- Tu, W.J.; Guo, M.; Shi, X.D.; Cai, Y.; Liu, Q.; Fu, C.W. First-Trimester Serum Fatty Acid-Binding Protein 4 and Subsequent Gestational Diabetes Mellitus. Obstet. Gynecol. 2017, 130, 1011–1016.
- Coe, N.R.; Bernlohr, D.A. Physiological properties and functions of intracellular fatty acid-binding proteins. Biochim. Biophys. Acta 1998, 1391, 287–306.
- Maeda, K.; Cao, H.; Kono, K.; Gorgun, C.Z.; Furuhashi, M.; Uysal, K.T.; Cao, Q.; Atsumi, G.; Malone, H.; Krishnan, B.; et al. Adipocyte/macrophage fatty acid binding proteins control integrated metabolic responses in obesity and diabetes. Cell. Metab. 2005, 1, 107–119.
- Kralisch, S.; Klöting, N.; Ebert, T.; Kern, M.; Hoffmann, A.; Krause, K.; Jessnitzer, B.; Lossner, U.; Sommerer, I.; Stumvoll, M.; et al. Circulating adipocyte fatty acid-binding protein induces insulin resistance in mice in vivo. Obesity (Silver Spring) 2015, 23, 1007–1013.
- Li, S.; Bi, P.; Zhao, W.; Lian, Y.; Zhu, H.; Xu, D.; Ding, J.; Wang, Q.; Yin, C. Prognostic Utility of Fatty Acid-Binding Protein 4 in Patients with Type 2 Diabetes and Acute Ischemic Stroke. Neurotox. Res. 2018, 33, 309–315.
- Furuhashi, M.; Tuncman, G.; Görgün, C.Z.; Makowski, L.; Atsumi, G.; Vaillancourt, E.; Kono, K.; Babaev, V.R.; Fazio, S.; Linton, M.F.; et al. Treatment of diabetes and atherosclerosis by inhibiting fatty-acid-binding protein aP2. Nature 2007, 447, 959–965.
- Erbay, E.; Babaev, V.R.; Mayers, J.R.; Makowski, L.; Charles, K.N.; Snitow, M.E.; Fazio, S.; Wiest, M.M.; Watkins, S.M.; Linton, M.F.; et al. Reducing endoplasmic reticulum stress through a macrophage lipid chaperone alleviates atherosclerosis. Nat. Med. 2009, 15, 1383–1391.
- Hui, X.; Li, H.; Zhou, Z.; Lam, K.S.; Xiao, Y.; Wu, D.; Ding, K.; Wang, Y.; Vanhoutte, P.M.; Xu, A. Adipocyte fatty acid-binding protein modulates inflammatory responses in macrophages through a positive feedback loop involving c-Jun NH2-terminal kinases and activator protein-1. J. Biol. Chem. 2010, 285, 10273–10280.
- Hotamisligil, G.S.; Bernlohr, D.A. Metabolic functions of FABPs--mechanisms and therapeutic implications. Nat. Rev. Endocrinol. 2015, 11, 592–605.
- Smith, A.J.; Thompson, B.R.; Sanders, M.A.; Bernlohr, D.A. Interaction of the adipocyte fatty acid-binding protein with the hormone-sensitive lipase: regulation by fatty acids and phosphorylation. J. Biol. Chem. 2007, 282, 32424–32432.
- Thompson, B.R.; Mazurkiewicz-Muñoz, A.M.; Suttles, J.; Carter-Su, C.; Bernlohr, D.A. Interaction of adipocyte fatty acid-binding protein (AFABP) and JAK2: AFABP/aP2 as a regulator of JAK2 signaling. J. Biol. Chem. 2009, 284, 13473–13480.
- Furuhashi, M.; Saitoh, S.; Shimamoto, K.; Miura, T. Fatty Acid-Binding Protein 4 (FABP4): Pathophysiological Insights and Potent Clinical Biomarker of Metabolic and Cardiovascular Diseases. Clin. Med. Insights Cardiol. 2015, 8, 23–33.
- Li, H.; Luo, H.Y.; Liu, Q.; Xiao, Y.; Tang, L.; Zhong, F.; Huang, G.; Xu, J.M.; Xu, A.M.; Zhou, Z.G.; et al. Intermittent High Glucose Exacerbates A-FABP Activation and Inflammatory Response through TLR4-JNK Signaling in THP-1 Cells. J. Immunol. Res. 2018, 2018, 1319272.
- Fu, Y.; Luo, N.; Lopes-Virella, M.F.; Garvey, W.T. The adipocyte lipid binding protein (ALBP/aP2) gene facilitates foam cell formation in human THP-1 macrophages. Atherosclerosis 2002, 165, 259–269.
- Kazemi, M.R.; McDonald, C.M.; Shigenaga, J.K.; Grunfeld, C.; Feingold, K.R. Adipocyte fatty acid-binding protein expression and lipid accumulation are increased during activation of murine macrophages by toll-like receptor agonists. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 1220–1224.
- Pelton, P.D.; Zhou, L.; Demarest, K.T.; Burris, T.P. PPARgamma activation induces the expression of the adipocyte fatty acid binding protein gene in human monocytes. Biochem. Biophys. Res. Commun. 1999, 261, 456–458.
- Yao, F.; Li, Z.; Ehara, T.; Yang, L.; Wang, D.; Feng, L.; Zhang, Y.; Wang, K.; Shi, Y.; Duan, H.; et al. Fatty Acid-Binding Protein 4 mediates apoptosis via endoplasmic reticulum stress in mesangial cells of diabetic nephropathy. Mol. Cell. Endocrinol. 2015, 411, 232–242.
- Nakamura, R.; Okura, T.; Fujioka, Y.; Sumi, K.; Matsuzawa, K.; Izawa, S.; Ueta, E.; Kato, M.; Taniguchi, S.I.; Yamamoto, K. Serum fatty acid-binding protein 4 (FABP4) concentration is associated with insulin resistance in peripheral tissues, A clinical study. PLoS ONE 2017, 12, e0179737.
- Zhang, Y.; Zhang, H.H.; Lu, J.H.; Zheng, S.Y.; Long, T.; Li, Y.T.; Wu, W.Z.; Wang, F. Changes in serum adipocyte fatty acid-binding protein in women with gestational diabetes mellitus and normal pregnant women during mid- and late pregnancy. J. Diabetes Investig. 2016, 7, 797–804.
- Xie, X.; Yi, Z.; Sinha, S.; Madan, M.; Bowen, B.P.; Langlais, P.; Ma, D.; Mandarino, L.; Meyer, C. Proteomics analyses of subcutaneous adipocytes reveal novel abnormalities in human insulin resistance. Obesity (Silver Spring) 2016, 24, 1506–1514.
- Ning, H.; Tao, H.; Weng, Z.; Zhao, X. Plasma fatty acid-binding protein 4 (FABP4) as a novel biomarker to predict gestational diabetes mellitus. Acta Diabetol. 2016, 53, 891–898.
- Nourani, M.R.; Owada, Y.; Kitanaka, N.; Sakagami, H.; Hoshi, H.; Iwasa, H.; Spener, F.; Kondo, H. Occurrence of immunoreactivity for adipocyte-type fatty acid binding protein in degenerating granulosa cells in atretic antral follicles of mouse ovary. J. Mol. Histol. 2005, 36, 491–497.
- Hsu, W.C.; Okeke, E.; Cheung, S.; Keenan, H.; Tsui, T.; Cheng, K.; King, G.L. A cross-sectional characterization of insulin resistance by phenotype and insulin clamp in East Asian Americans with type 1 and type 2 diabetes. PLoS ONE 2011, 6, e28311.
- Ota, H.; Furuhashi, M.; Ishimura, S.; Koyama, M.; Okazaki, Y.; Mita, T.; Fuseya, T.; Yamashita, T.; Tanaka, M.; Yoshida, H.; et al. Elevation of fatty acid-binding protein 4 is predisposed by family history of hypertension and contributes to blood pressure elevation. Am. J. Hypertens. 2012, 25, 1124–1130.
- Wu, L.E.; Samocha-Bonet, D.; Whitworth, P.T.; Fazakerley, D.J.; Turner, N.; Biden, T.J.; James, D.E.; Cantley, J. Identification of fatty acid binding protein 4 as an adipokine that regulates insulin secretion during obesity. Mol. Metab. 2014, 3, 465–473.
- Greco, E.A.; Francomano, D.; Fornari, R.; Marocco, C.; Lubrano, C.; Papa, V.; Wannenes, F.; di Luigi, L.; Donini, L.M.; Lenzi, A.; et al. Negative association between trunk fat, insulin resistance and skeleton in obese women. World J. Diabetes 2013, 4, 31–39.
- Ni, X.; Gu, Y.; Yu, H.; Wang, S.; Chen, Y.; Wang, X.; Yuan, X.; Jia, W. Serum Adipocyte Fatty Acid-Binding Protein 4 Levels Are Independently Associated with Radioisotope Glomerular Filtration Rate in Type 2 Diabetic Patients with Early Diabetic Nephropathy. Biomed. Res. Int. 2018, 2018, 4578140.
- Toruner, F.; Altinova, A.E.; Akturk, M.; Kaya, M.; Arslan, E.; Bukan, N.; Kan, E.; Yetkin, I.; Arslan, M. The relationship between adipocyte fatty acid binding protein-4, retinol binding protein-4 levels and early diabetic nephropathy in patients with type 2 diabetes. Diabetes Res. Clin. Pract. 2011, 91, 203–207.
- Yeung, D.C.; Xu, A.; Tso, A.W.; Chow, W.S.; Wat, N.M.; Fong, C.H.; Tam, S.; Sham, P.C.; Lam, K.S. Circulating levels of adipocyte and epidermal fatty acid-binding proteins in relation to nephropathy staging and macrovascular complications in type 2 diabetic patients. Diabetes Care 2009, 32, 132–134.
- Kralisch, S.; Stepan, H.; Kratzsch, J.; Verlohren, M.; Verlohren, H.J.; Drynda, K.; Lössner, U.; Blüher, M.; Stumvoll, M.; Fasshauer, M. Serum levels of adipocyte fatty acid binding protein are increased in gestational diabetes mellitus. Eur. J. Endocrinol. 2009, 160, 33–38.
- Cabré, A.; Lázaro, I.; Girona, J.; Manzanares, J.M.; Marimón, F.; Plana, N.; Heras, M.; Masana, L. Plasma fatty acid binding protein 4 is associated with atherogenic dyslipidemia in diabetes. J. Lipid Res. 2008, 49, 1746–1751.
- Okazaki, Y.; Furuhashi, M.; Tanaka, M.; Mita, T.; Fuseya, T.; Ishimura, S.; Watanabe, Y.; Hoshina, K.; Akasaka, H.; Ohnishi, H.; et al. Urinary excretion of fatty acid-binding protein 4 is associated with albuminuria and renal dysfunction. PLoS ONE 2014, 9, e115429.
- Tanaka, M.; Furuhashi, M.; Okazaki, Y.; Mita, T.; Fuseya, T.; Ohno, K.; Ishimura, S.; Yoshida, H.; Miura, T. Ectopic expression of fatty acid-binding protein 4 in the glomerulus is associated with proteinuria and renal dysfunction. Nephron. Clin. Pract. 2014, 128, 345–351.
- Hu, X.; Ma, X.; Luo, Y.; Xu, Y.; Xiong, Q.; Pan, X.; Bao, Y.; Jia, W. Contribution of serum adipocyte fatty acid-binding protein levels to the presence of microalbuminuria in a Chinese hyperglycemic population. J. Diabetes Investig. 2017, 8, 582–589.
- Scalera, A.; Tarantino, G. Could metabolic syndrome lead to hepatocarcinoma via non-alcoholic fatty liver disease? World J. Gastroenterol. 2014, 20, 9217–9228.
- Thompson, K.J.; Austin, R.G.; Nazari, S.S.; Gersin, K.S.; Iannitti, D.A.; McKillop, I.H. Altered fatty acid-binding protein 4 (FABP4) expression and function in human and animal models of hepatocellular carcinoma. Liver Int. 2018, 38, 1074–1083.
