1. Introduction
Advances in biology, medicine and genetics have led today to a better understanding of diseases and their genetic nature. These advances have also given rise to innovations in drug research and development as well as delivery technologies for therapeutics.
Consecutively, the idea of treating the underlying factor of the disease by modulating disease-causing gene activity instead of by using the classical approach of treating the symptoms of the disease with conventional drugs has started to revolutionize healthcare. This gene-based therapeutic approach, known as gene therapy, can be used to treat not only diseases caused by genetic disorders but also acquired diseases such as infectious diseases or cancer [
1]. Gene therapy, using, among other treatments, plasmid DNA (pDNA), messenger RNA (mRNA), antisense oligonucleotides (ASOs) and small interfering RNA (siRNA), has shown great potential compared to conventional therapies and shows huge potential to be used in clinic. However, the clinical translation of nucleic acids is challenging because of their hydrophilicity, high molecular weight, fragile structures susceptible to in vivo degradation, and low stability, as well as the difficulty involved in reaching the site of action. Thus, they require a delivery system that can safely and efficiently transport them to the target site [
2].
Indeed, the determination of the nucleic acid carrier vector is the most important point when designing gene delivery systems. In the literature, carrier vectors used for gene therapy are classified into two main categories: viral and non-viral. As the name indicates, viral systems consist of viruses in their non-pathogenic form, which have been modified to be replication-deficient. Adenoviruses, adenovirus-associated viruses, retroviruses, lentiviruses and bacteriophages are widely used as viral gene delivery vectors. However, viral vectors show disadvantages in terms of the induction of unwanted immune responses and are characterized by limited packaging capacity [
3,
4].
Non-viral gene delivery systems have been developed as an alternative to viral systems. The most important advantages of these systems are their enhanced safety profile and their large packaging capacity for genetic material [
5]. Non-viral methods for gene transfer are divided into physical and chemical subgroups. Physical methods consist of gene gun, microinjection via needles, laser irradiation, electroporation and sonoporation techniques [
6]. On the other hand, chemical methods can be categorized as organic and inorganic. The organic category includes carrier systems such as polymeric micelles, dendrimers and liposomes [
7], while the inorganic category consists of delivery systems such as the silver nanoparticles or magnetic nanoparticles of inorganic materials [
6].
Micelles are widely used as non-viral systems in gene therapy [
8]. Biocompatibility, ease of preparation, the opportunity to adjust particle size, good long-term stability and the possibility to control their physicochemical characteristics are the most important factors in the preference of micelles [
9]. The history of using micelles as a carrier system in gene therapy goes back to the 1980s. Since the first years of its use, micelles have attracted increasing attention as versatile delivery vehicles for nucleic acids.
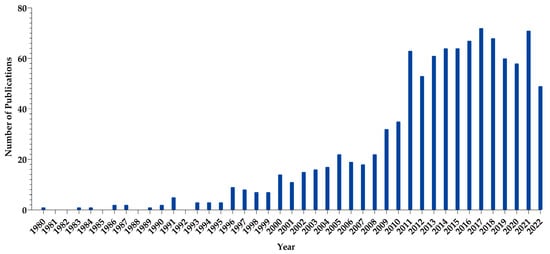
Figure 1 displays the PubMed database of studies related to micelle-based systems developed for the purpose of gene therapy [
10].
Figure 1. Micelle-related articles published from 1980 to 2022. (Data gathered from PubMed using the keywords micelle, plasmid DNA, antisense nucleotide, mRNA and siRNA).
Micelles are commonly defined as self-assembled structures of amphiphilic molecules above a specific minimal concentration known as the critical micelle concentration (CMC). While micelles are characterized by a hydrophobic inner core and a hydrophilic outer shell, structures named “reverse micelles” or “inverse micelles’’ consisting of a hydrophilic core and a hydrophobic shell can also be formed [
11]. Although the term “micelle” is widely used in the literature to indicate, in general, the formation of core–shell micellar structures, polymeric micelles, specifically, are nano-sized carrier systems that are formed by the self-assembly of amphiphilic blocks (di- or tri-) or graft polymers above the CMC. Polymeric micelles can incorporate and transport the lipophilic agents in the hydrophobic inner core and hydrophilic agents in the hydrophilic outer shell. Compared to micellar structures composed of conventional surfactant molecules, i.e., small molar mass molecules, polymeric micelles are formed at lower CMC values and tend to be more stable in vitro and in vivo [
12,
13]. For this reason, polymeric micelles are widely used in studies as nanocarrier systems for the delivery of drugs and other therapeutic agents.
The polymers used in the preparation of polymeric micelles can be natural or synthetic [
14]. Owing to the development of polymerization technology, copolymers with different structures and desired physicochemical properties can be synthesized using various hydrophilic, hydrophobic and cationic blocks [
8,
15]. Thus, the structural properties of polymeric micelles, such as their surface charge and size and the release mechanism of the therapeutics, can be altered to design effective delivery systems and optimize other factors, such as cellular uptake, endosomal escape and cellular release, that influence treatment efficacy [
13]. Furthermore, polymeric micelles offer the opportunity to delivery both drugs and nucleic acid therapeutics simultaneously. Therefore, they have been gaining increased interest to achieve synergistic combination treatment particularly with targeted cancer treatment [
16]. These advantages have made polymeric micelles an important non-viral delivery system for nucleic acids (NA).
2. Polymeric Micelles for Nucleic Acid Delivery
2.1. Polymers and Modifying Agents in Polymeric Micelles
The most critical point in the preparation of PMs with optimal characteristics is the selection of the right polymers and modifying agents. Many characteristic features of the PMs discussed above are directly dependent on the properties and structure of the polymer/modifying agent [
13].
There are various types of polymers and modifying agents investigated for the preparation of NA-carrying PMs in studies present in the literature. Table 1 and Table 2 summarize these studies, including the polymers, the type of nucleic acid that is delivered, their application, and the characteristics of the polymer or modifying agent or the type of stimuli-responsiveness.
Table 1. Polymers/modifying agents used in the preparation of NA-carrying PMs, the type of NA and the target disease category.
| Polymer/Modifying Agent |
Characteristic |
Nucleic Acid |
Disease Category |
References |
| Aliphatic chain |
Hydrophobic |
siRNA |
Oncology |
[130] |
| Amines |
Cationic |
siRNA |
Oncology |
[130] |
| APA |
Amphiphilic |
siRNA |
Oncology |
[130] |
| APD |
Cationic |
pDNA |
Oncology |
[131] |
| Cholic acid |
Hydrophobic |
pDNA |
Oncology |
[131] |
| CCP |
Anionic |
siRNA |
Oncology |
[132] |
| CPP |
Cationic |
miRNA |
Oncology |
[133] |
| siRNA |
Oncology |
[134,135] |
| Cys-bridged His-Arg |
Cationic |
siRNA |
Oncology |
[136] |
| DOTAP |
Cationic |
siRNA |
Oncology |
[137] |
| |
|
pDNA |
Oncology |
[138] |
| DP |
Cationic |
siRNA |
Oncology |
[139] |
| EMA |
Cationic |
siRNA |
Oncology |
[140] |
| MAODCA |
Hydrophobic |
pDNA |
Oncology |
[141] |
| NVP |
Cationic |
mRNA |
- |
[142] |
| PAGA |
Cationic |
siRNA |
Oncology |
[143] |
| pAsp |
Cationic/Hydrophobic |
mRNA |
Neurology |
[95] |
| siRNA |
Viral |
[144] |
| siRNA |
Oncology |
[134,135,145] |
| - |
Orthopedia |
[146] |
| PCC |
Cationic |
miRNA |
Oncology |
[147] |
| PCL |
Hydrophobic |
siRNA |
Oncology |
[137,139,148,149,150,151,152,153,154,155] |
| pDNA |
Oncology |
[138,156] |
| pCys |
Hydrophobic |
miRNA |
Oncology |
[157] |
| PDMAEMA |
Cationic |
siRNA |
Oncology |
[148,149,155,158] |
| pDNA |
- |
[141,159] |
| PDPA |
Cationic/Hydrophobic |
siRNA |
Oncology |
[143] |
| pDNA |
Oncology |
[159] |
| PE |
Hydrophobic |
siRNA |
Oncology |
[43] |
| PEG/mPEG/PEO |
Hydrophilic |
pDNA |
Oncology |
[138,156,160] |
| siRNA |
Oncology |
[43,134,135,136,137,140,143,145,150,151,152,153,154,155,161,162,163,164,165] |
| miRNA |
Oncology |
[133,147,157] |
| mRNA |
Neurology |
[95] |
| - |
Orthopedia |
[146] |
| PEI |
Cationic |
pDNA |
Neurology |
[147] |
| pDNA |
Oncology |
[156,166] |
| siRNA |
Oncology |
[150,163,167] |
| PGA |
Hydrophobic |
siRNA |
Oncology |
[140] |
| PHB |
Amphiphilic |
siRNA |
Oncology |
[158] |
| PHis |
Hydrophobic |
| PLA |
Hydrophobic |
mRNA |
- |
[142] |
| miRNA |
Oncology |
[133] |
| PLGA |
Hydrophobic |
pDNA |
Neurology |
[147] |
| siRNA |
Oncology |
[164,167] |
| PLL |
Cationic/Hydrophobic |
siRNA |
Oncology |
[152,164] |
| miRNA |
Oncology |
[157] |
| siRNA |
Oncology |
[145,168] |
| PMPMC |
Hydrophobic |
siRNA |
Oncology |
[161] |
| P(NAS-co-NVP) |
Amphiphilic |
mRNA |
- |
[142] |
| POEOMA |
Hydrophobic |
pDNA |
Oncology |
[159] |
| Polystyrene |
Hydrophobic |
siRNA |
Oncology |
[136] |
| PPA |
Cationic |
siRNA |
Oncology |
[162] |
| PPEEA |
Cationic |
siRNA |
Oncology |
[153,154] |
| PSMA |
Amphiphilic |
siRNA |
Oncology |
[136] |
| RGD |
Amphiphilic |
siRNA |
Oncology |
[168] |
| SP |
Cationic |
siRNA |
Oncology |
[139] |
| TAT |
Cationic |
siRNA |
Oncology |
[151] |
| TEPA |
Hydrophobic |
miRNA |
Oncology |
[147] |
| TP |
Cationic |
siRNA |
Oncology |
[139] |
| TPP |
Hydrophobic |
pDNA |
Oncology |
[166] |
Table 2. Stimuli-responsive polymers used in the preparation of PMs and the corresponding type of NA.
| Stimuli-Responsiveness |
Functional Vector |
Nucleic Acid |
References |
| pH-responsive |
PLL-polyhistidine |
siRNA |
[169] |
| Poly(styrene-alt-maleic anhydride) |
DNA |
[170] |
| Cross-linked low Mw PEI by imine linkers |
DNA |
[171] |
| Lactosylated PEG–PLL |
siRNA |
[172] |
| Ketalized PEI |
DNA/siRNA |
[173] |
| ROS-responsive |
PEG–thiolated PLL |
DNA |
[174] |
| PEG–thiolated PLL |
siRNA |
[175] |
| PEG–thiolated PLL–melittin–siRNA |
siRNA |
[176] |
| PEI–PHPMA |
DNA |
[177] |
| Cyclodextrins threaded onto PEG |
DNA |
[178] |
| Enzyme-responsive |
PEI-FPBA/Chol-DOPA |
siRNA |
[179] |
| PEG-PLG∗LAGr9–PCL |
siRNA |
[180] |
| PEG-pp-PEI-PE |
siRNA |
[181] |
| Temperature-responsive |
PEI–poly(NIPAM–acrylamide)/PEI–poly(NIPAMvinylpyrrolidone) |
DNA |
[182] |
| ATP-responsive |
FPBA-functionalized/PEG-PLL |
siRNA |
[183] |
| PEG-PBA |
mRNA |
[184] |
2.2. Plasmid DNA (pDNA) Delivery
Plasmids are extrachromosomal, circular double-stranded DNA molecules that are naturally present mainly in bacteria, but they can also be found in other microorganisms. Plasmid DNA (pDNA) can be genetically engineered to carry genes for encoding specific proteins. They are used for gene therapy to treat or cure several diseases including genetic disorders and cancer, as well as for vaccination [
185]. The introduction of therapeutic genes into the cell nucleus can modify gene expression by replacing, inactivating or introducing a particular gene. pDNA is easy to produce, but its delivery to the target cell is still one of the biggest challenges for gene therapy because of rapid enzymatic degradation upon administration and poor transfection, particularly in nondividing cells, which results in limited efficacy [
186]. Although pDNA can be delivered simply in its free form as naked DNA, PM-mediated pDNA delivery can provide DNA condensation and protection from degradation, promote both cellular uptake and nuclear delivery, and target release.
It was reported in the early 1990s that the incorporation of plasmids into soluble interpolyelectrolyte complexes, which formed spontaneously due to the electrostatic interaction between DNA and quaternized poly (4-vinylpyridines), enhanced DNA penetration into the cell and in vitro cell transforming efficiency [
187,
188]. Since then, the understanding of pDNA delivery and transfection efficacy has increased, and different types of cationic polymers have been investigated for the preparation of PMs incorporating pDNA. Among others, PEI, PLL, PAMAM, poly(methacrylate) and chitosan-based micelles have been widely investigated for gene therapy. In this part, the PMs of various polymers that have been explored for the delivery of pDNA are discussed.
PEI is an organic cationic polymer that can be either in linear or branched forms. It is one of the earliest and most studied polymers and has been successfully used for the delivery of pDNA. It is often referred to as the “gold standard” for non-viral vectors due to its high transfection efficacy [
189]. However, the molecular weight and structure of PEI affects the performance of PMs. Despite showing better stability and transfection activity, high-molecular-weight PEI induces greater cytotoxicity, whereas PEI with a low molecular weight shows lower transfection activity even though it is more cytocompatible [
190]. Recognizing this, modifying PEI with different polymers and groups has been intensely investigated to prolong its systemic circulation and overcome toxicity, aggregation, precipitation and stability limitations when used as polycation transfectant. Among others, grafting PEI with PEG of different molecular weight and structures is extensively studied to enhance the in vitro and in vivo gene expression. Nevertheless, an optimal degree of PEG grafting is essential [
191]. Velluto et al. [
192] synthesized a triblock copolymer, poly(ethylene glycol)-
b-poly(propylene sulfide)-
b-poly(ethylene imine) (PEG-b-PPS-
b-PEI), and reported that PEG-
b-PPS-
b-PEI micelles and the PEG-
b-PPS/PEG-
b-PPS-
b-PEI micelle demonstrated good transfection of pDNA in tumor cells in vitro and in vivo after intratumoral injections while showing markedly reduced cytotoxicity compared to that of linear PEI alone, 10 kDA. Recently, Abd Elhameed et al. [
193] showed that cancer cells were efficiently transfected by high-molecular-weight-PEI-based water-soluble lipopolymer containing EGFP-encoding plasmids. While both the investigated dose and cell line affected the toxicity, significant toxicity was not observed at concentrations as high as ≈150 ng per well in A549 and HeLa cells [
193]. On the other hand, the modification of low-molecular-weight PEI with either α-tocopherol, cholesterol or diosgenin showed that the polymers with lipophilic parts could form micelles and demonstrated higher transfection efficacy compared to 25-kDa PEI [
194].
In another study, pEGFP-C3 plasmid DNA was successfully condensed in polymeric micelles prepared with partially hydrolyzed poly(2-ethyl-2-oxazoline)-
co-poly(ethyleneimine)-
block-poly(
ε-caprolactone), which showed a low CMC, good serum stability and high transfection efficacy of MCF-7 and MDAMB-468 cells [
195]. In a more recent study, the potential of magnetic polymeric micelles for targeted drug delivery both for diagnosis and therapeutic purposes in MCF-7 cells was also investigated [
196]. Spherical FePECLEFE/DNA micelles (Fe
3O
4-PEI-polycaprolactone (FePEC)/folic acid (FA)- polyethylene glycol (PEG)- polyethyleneimine (PEI)-polylactic acid (PLA) (FA-PEG-PEI-PLA-PEI-PEG-FA) (PLEEF)/EPPT peptide (FePECLEFE) micelles) with a particle size of about 200–300 nm not only had good biocompatibility but also showed a high ability to neutralize DNA and protect it against restriction enzymes, resulting in high gene transfer efficiency. In addition, flow cytometry results revealed that micelles prepared at a 10:1:0.5:1 FePEC/PLEEF/EPPT/DNA mass ratio (w/w/w/w %) had the highest gene transfer efficiency in MCF-7 cells in serum-containing and serum-free media. It was also emphasized that, when micelles containing folic acid, which is known to be absorbed into cancer cells by binding to folic acid receptors and endocytosis, were used, the gene transfer efficiency of pEGFP-N1 increased, making these polymeric micelles particularly attractive for use as a theragnostic [
196]. In another study, Garg et al. [
197] reported the design and synthesis of an amphiphilic cationic polymer–peptide conjugate from a low-molecular-weight PEI (1.8 kDa) and a synthetic peptide, which self-assembled to form positively charged micelles of ~144–205 nm, which varied according to the amount of peptide used. They were cytocompatible while showing comparable transfection of HEK 293 cells with Lipofectamine/pDNA complexes [
197].
Poly-L-lysine is one of the poly(amino acids) widely investigated for gene therapy because it is not only positively charged but also contains many active side chain groups. For example, by the reaction of the carboxylic terminal end group of PLGA and the amine group in PLL, amphiphilic graft copolymer PLGA-grafted PLL were synthesized. The preparation of PLGA-grafted PLL micelle/DNA complexes with sizes between 200 and 300 nm and with a positive surface charge demonstrated a transfection efficiency that was about 10-fold higher for pRSVLuc compared to PLL while showing five times lower cytotoxicity, which was probably due to the lower charge density of the PLL micelles [
198]. On the other hand, when two cysteines were separately allocated in PEG−oligolysines with 15 or 20 amino acid and cross-linked PMs incorporating luciferase-coding pDNA were formulated, a relationship between the peptide sequence and in vitro gene expression was demonstrated. Although high gene expressions were seen for both PEG−oligolysines with 15 or 20 amino acids in cell-free assays, only micelles containing 20 amino acids showed significant expressions in the cell-based assay in HeLa cells. Additionally, a cysteine addition was required for the stabilization of PEG−peptide PMs via disulfide crosslinks [
199]. Another study also described the crucial role of disulfide crosslinking into the poly(ethylene glycol)-
b-poly(l-lysine) micelle cores to increase the stability of micelles loaded with pDNA encoding an anti-angiogenic protein (sFlt-1) against shear stress in the blood stream and improve their in vivo blood circulation [
200].
Polymeric micelles prepared with PAMAM dendrimers have also been proven to be efficient gene transfection vectors for pDNA. While PAMAMs of low generations (G < 3) are easy to synthesize and less toxic in contrast to PAMAMs of high generations (G > 5), their low transfection efficacy does not provide adequate treatment. Tuning the structure of PAMAMs by introducing different groups and altering the physicochemical properties of polymers has been reported to improve the performance of PAMAM-based dendrimers in vitro and in vivo in different studies [
201,
202]. Piao et al. [
203] added a RAGE-antagonist peptide (RAP) to dexamethasone-conjugated polyamidoamine G2 (PAM-D) with the aim of facilitating the PM-mediated intracellular delivery of an APN plasmid for the treatment of acute lung injury. As expected, it was reported that pAPN was successfully delivered in vitro into the L2 cells and that pAPN/PAM-D/RAP had high therapeutic effects in an acute lung injury mouse model, which was attributed to the synergistic effects of RAP and PAM-D. In another study [
204], the authors demonstrated that the combined delivery of curcumin loaded into cholesterol-conjugated polyamidoamine PMs and further complexed with the heme oxygenase-1 gene improved gene delivery efficiency and showed greater anti-inflammatory effects in lungs compared to curcumin or plasmid heme oxygenase-1 alone. It was reported that curcumin, which is a hydrophobic drug, can be loaded into the core of the micelles, whereas the plasmid can be complexed via the positive charge on the surface of the micelle [
204]. The functionalization of cholesterol-conjugated histidine- and arginine-grafted polyamidoamine PMs with glycyrrhizic acid also could be promising for gene therapy for inflammatory lung diseases [
115].
Other PM-based delivery systems, such as T7-conjugated redox-sensitive amphiphilic micelles using polyethylene glycol-polyethyleneimine-poly(caprolactone)-S-S-poly(caprolactone)-polyethyleneimine-polyethylene glycol, which are used to treat breast cancer [
205], and chitosan-based micelles for enhanced cellular immunity [
206], have also been designed, and their potential to deliver pDNA has been investigated.
2.3. Messenger RNA (mRNA) Delivery
The use of mRNA for preventing or treating numerous diseases has emerged as a promising strategy for several decades. Apparently, the introduction of mRNA-based vaccines in clinics in late 2020 has accelerated even more the development of mRNA therapeutics. Basically, mRNA delivers genetic information into cells where it is translated into a functional protein in cytoplasm [
207]. Despite their potential to treat challenging diseases, ease of production, scalability and lack of potential risk of integration with the natural host genome, mRNAs are rapidly degraded by nucleases and show low stability and poor cellular uptake. For successful translation to occur, mRNA should reach the cellular machinery intact. Apart from the rational design of mRNA sequences to increase stability and enhance translational efficiency, different delivery vehicles are being extensively investigated. Even though cationic lipids have been in the spotlight of extensive research to effectively protect and transport mRNA to cells, great interest has been placed on the use of polymers as versatile agents with tunable properties for safe and efficient delivery [
208].
The use of polymeric micelles as delivery vectors for mRNA has shown promising outcomes both in vitro and in mouse models against various diseases including neurological disorders, cancer immunotherapy and vaccination [
209,
210,
211,
212]. To achieve maximum protein expression, the modulation of micelle properties by different approaches has attracted various investigations. While PEI polymers have been commonly explored for the delivery of mRNA too, its wide therapeutic application remains challenging due to its toxicity, as mentioned previously. Other frequently studied polymers for mRNA delivery are polymethacrylates [
213], amino-polyesters, PLL and PAMAM dendrimers [
214].
Among other methods, the incorporation of hydrophilic segments, usually PEG chains, into the structure of polymers has been shown to be beneficial for reducing unwanted responses and increasing circulation time and therapeutic efficacy. PEGylated PM nanomicelles of 24 and 34 nm containing four repeating units of aminoethylene groups appeared to elicit low levels of proinflammatory cytokines following intracerebroventicular delivery while providing mRNA protection in a mouse model [
215]. However, PEG shielding should be optimized [
211]. PEGylated PM demonstrated successful in vivo genome editing in mouse brains. In the corresponding study, Cas9 mRNA and sgRNA of 4.5 kb and 0.1 kb, respectively, were co-delivered using poly(
N’-(
N-(2-aminoethyl)-2-aminoethyl) aspartamide as the polycationic segment, emphasizing that PEG was required for effective genome editing [
95]. mRNA nanomicelles prepared with polyethylene glycol-poly(
N’-(
N-(2-aminoethyl)-2-aminoethyl)aspartamide) block copolymer have been demonstrated to be attractive options for the treatment of spinal cord injury in a mouse model when brain-derived neurotrophic factor mRNA was delivered [
216].
Efforts have also been made to improve outcomes of PM-based delivery for gene therapy via hydrophobic modification for the tuning characteristics of hydrophilic polymers. Modifying PEI (1.8 kDa) with vitamin E succinate, which could form self-assembled micelles with an average size of 144.7 ± 0.76 nm at a 32 N/P ratio for mRNA vaccine delivery, resulted in the successful transfection of HeLa, HEK-293T, Vero and DC2.4 cells while exhibiting much lower cytotoxicity compared to the positive control PEI 25k [
210]. Also, the synthesis of a stearic acid–PEI copolymer and the formation of self-assembled cationic nanomicelles were shown to improve anti-HIV1 gag-specific immune responses when in vitro transcribed gag mRNA was delivered [
217].
Another approach used to enhance the protein expression of mRNA-loaded polymeric micelles both in vitro and in vivo is the use of stimuli-responsive micelles. For this purpose, the introduction of degradable bonds, such as ester and disulfide, is an attractive approach. Yang et al. designed pH sensitive cross-linked micelles of
cis-aconitic anhydride-modified poly(ethylene glycol)-poly (L-lysine) (PEG-PLL(CAA)) block copolymers that were capable of releasing mRNA triggered by endosomal pH (pH 5.5–4.5) while remaining stable at physiological pH and protecting mRNA from enzymatic degradation [
209].
More recently, polymeric micelles based on block copolymer poly(ethylene glycol)-poly(glycerol) (PEG-PG) modified with either glycine (Gly), leucine (Leu) or tyrosine (Tyr) by the formation of ester bonds were investigated for in vivo delivery. In particular, PEG-PG modified with Tyr provided excellent mRNA protection in serum and higher cellular uptake in Huh7 cells. Moreover, mRNA integrity in blood was prolonged after i.v. administration compared to Gly- and Leu-modified micelles. Additionally, when micelles containing firefly luciferase mRNA were evaluated, strong bioluminescent signals were observed making PEG-PGTyr micelles attractive carriers for mRNA delivery [
218].
While extensive research in the literature report the delivery of mRNA by local or parenteral injection, current research studies are also being directed towards the administration of mRNA via non-invasive routes. In this content, the inhalable mRNA PMs of hyperbranched poly(beta amino esters) showed localized delivery to the lungs without demonstrating local or systemic toxicity after repeated administrations [
219].
The delivery of mRNA using micelles based on cationic lipids and diblock polymers has also been reported to show promising results. For the treatment of colorectal cancer, the biodegradable micelles of DOTAP-poly(ethylene glycol)–poly(
ε-caprolactone) with a size of 30 nm could efficiently delivery mRNA on C26 mouse colon cancer cells (60.59%) and were effective and safe following systemic administration [
220]. Lately, the delivery of mRNA using a combination of lipid- and polymer-based nanoparticles has been explored as an attractive alternative for ornithine transcarbamylase deficiency. When mRNA encoding for ornithine transcarbamoylase (OTC) was administered to mice by the i.v. route, efficient protein production was observed in liver, suggesting that, while lipid nanoparticles protect the mRNA from nucleases, di-block PMs provide specific targeting to the liver and promote the endosomal release of mRNA [
221].
It can be concluded that, although most of the non-viral delivery vehicles that are currently in clinical trials investigating mRNA delivery particularly for cancer immunotherapy and vaccination are primarily based on lipid particles, rationally designed polymeric micelles also seem to be attractive candidates that can effectively protect and transport mRNA to cells.
2.4. Antisense Oligonucleotide (ASO) Delivery
Antisense oligonucleotides, shortly abbreviated as ASOs, are short, synthetic, single-stranded nucleic acids that can recognize and bind to a specific mRNA, thus modulating gene expression. Despite the increasing interest in ASO therapies, particularly for the treatment of genetic diseases, obstacles to efficient delivery to the cell remain to be overcome. While approaches such as the chemical modification of ASOs are based on the chemical modification of the structure to maintain stability and functionality as they reach cytoplasm, the use of PM-based systems can protect ASOs in the bloodstream and provide opportunity for manipulating delivery at the target site, simultaneously.
Various studies report the incorporation of ASOs in polymeric micelles that have been stabilized via different approaches. For example, Kakizawa et al. [
222] synthesized glutathione-sensitive thiolated poly(ethylene glycol)-
block-poly(l-lysine) that formed micelles crosslinked by disulfide bonds in their inner core, which not only enhanced the stability of the entrapped antisense sequence for vascular endothelial growth factor against nuclease but also improved its intracellular delivery. As an alternative to disulfide crosslinks, polyion complex micelles prepared with triblock copolymers composed of parts with different unique characteristics in terms of hydrophilicity–hydrophobicity–cationic charge can also be utilized for systemic ASO delivery to solid tumors [
223]. Notably, the polymer architecture and the presence of cationic moieties in polymers capable of forming PMs play a crucial role for ASO delivery [
224].
The use of ASO-loaded micelles could also be promising for systemic brain delivery, despite the presence of the blood–brain barrier (BBB) preventing the penetration of free molecules. The development of glucose-modified polyion complex micelles from poly(ethylene glycol)-
b-poly(l-lysine) modified with 3-mercaptopropyl amidine and 2-thiolaneimine block copolymers with a size smaller than 50 nm showed efficient accumulation in the brain [
225]. Glucose presence could aid the active translocation of the nanocarrier in the BBB via glucose-transporter 1 (GLUT1). ASO-loaded PM-based formulations demonstrated half-lives of 80–100 min in blood circulation compared to 9 min for naked ASO regardless of glucose numbers in their structure. Also, they showed enhanced cellular uptake. Nevertheless, the glucose number was shown to affect MALAT1 knockdown efficiency, with the highest efficiency obtained when 52 glucose molecules were used [
225].
Furthermore, ASO–polymer conjugate micelles have emerged in the literature demonstrating distinctive characteristics for gene silencing. Fakhoury et al. [
226] showed that when novel ASO–polymer conjugates able to associate into micelles and further complexed with 25 kDa linear PEI (HE
12-Luc-ASO) were used, significant firefly luciferase knockdown activity was successfully achieved. On the contrary, when ASO was conjugated to a polymer of similar length but with hexaethylene glycol-dodecane units (HE-HEG)
6-Luc-ASO) that did not form micelles, adequate gene silencing activity was not observed. Herein, using low concentrations of PEI, it was possible to obtain significant transfection and gene knockdown, maintaining minimal cytotoxicity [
226].
A similar approach for the intratracheal delivery of ASO to lung cancer was reported recently. When thermoresponsive poly(2-n-propyl-2-oxazoline) of 30k was conjugated with ASO to target taurine-upregulated gene 1 long noncoding RNA (TUG1 lncRNA), a gene which is frequently overexpressed in lung cancers, structures as small as 50 nm with a narrow size distribution (PDI:0.08) were formed. These conjugates reduced the expression level of TUG1 lncRNA at around 55% in the tumor at a dose of 15 µg. Interestingly, significant knockdown activity was not observed for non-conjugated ASO even though it was reported that considerable accumulation was observed in the lung and tumor [
227].
Apart from therapeutic applications, the potential of theragnostic micelles co-delivering siRNA/ASO for neural stem cell (NSC) therapy for ischemic stroke has shown encouraging results [
228]. Regardless of the benefits NCS treatments seem to offer in clinic, the differentiation of exogenous NSCs into neurons is limited [
229]. Hence,
Pnky lncRNA silencing, which appears to act as an inhibitor of the neuronal differentiation of NSCs, could enhance NSC-based therapy for stroke. MRI-visible nanocarriers composed of a cationic amphipathic polymer (PAsp(DMA)-Lys-(CA)
2) based on aspartate, lysine and cholic acid and superparamagnetic iron oxide nanoparticles (SPIO) self-assembled into cationic micelles loaded with siRNA/ASO at an optimum N/P ratio of 7/1 showed up to 95.88% in vitro transfection efficiency. Also, 63.2%
Pnky knockdown efficiency was observed at 24 h after transfection. In addition, an in vivo histological analysis demonstrated that a 5.5-fold increase in neuronal differentiation was achieved for
Pnky-targeted siRNA/ASO-loaded micelles 2 weeks after intracerebral transplantation in vivo in mice [
228].
All the successful outcomes obtained from the studies involving the use of polymeric micelle-based delivery address the beneficial aspects of these systems for the clinical translation of ASO therapies.
2.5. Small Interfering RNA (siRNA) Delivery
Small interfering RNAs (siRNA) are the cleavage products of dsRNA that can induce the deliberate silencing of protein coding genes. They are also named silencing RNA or short interfering RNA. The use of synthetic siRNA therapeutics for challenging diseases, particularly for various types of cancers and genetic diseases, has been a fast-growing area of research following the introduction of the RNA interference (RNAi) concept for gene silencing in early 1998 and the approval of the first siRNA therapeutic (patisiran) in 2011 for an orphan disease, hereditary transthyretin (hATTR) amyloidosis [
230]. Polymers generally investigated for gene delivery are also studied for the development of PMs for siRNA. Some of the most explored synthetic polymers since early studies are PEI [
231,
232], PLL [
233], poly(amido amine) [
234,
235] and PCL [
137,
149]. Furthermore, pH-responsive polymers are also widely explored for the rational design of PMs for siRNAs [
236,
237].
In line with previous studies reporting the successful delivery of nucleic acids (i.e., pDNA) by the lipid modification of PMs, the delivery of siRNA via lipid (DOTAP)-modified monomethoxy poly(ethylene glycol)-poly(
ε-caprolactone) (MPEG-PCL) hybrid cationic PMs for colon cancer therapy was efficacious. Anticancer activity seen in vitro could be due to the inhibition of the proliferation of C26 cells. Also, gene silencing was obtained in vivo [
137]. In another study, the peptide modification approach was used to prepare CH2R4H2C-peptide-modified MPEG-PCL nanomicelles (~60 nm) delivering the NF-κB-targeting siRNA (siRelA) gene for effective treatment. After i.v. administration in a mice model, anti-inflammatory activity against ulcerative colitis was seen, which was also characterized by a decrease in the shortening of an inflamed large intestine, clinical score and inflammatory cytokine production [
238].
PMs could be interesting candidates for synergistic therapy against cancer to co-deliver siRNA with chemotherapeutic small molecule drugs. Li et al. synthesized an amphiphilic block copolymer to prepare a pH-sensitive micelle having PEG in the shell to increase in vivo circulation, PLL to facilitate siRNA loading and poly(aspartyl(benzylamine-co-(diisopropylamino)ethylamine) in the hydrophobic core for enhancing stability and pH sensitivity. Positively charged PMs of a small size (~70 nm) successfully co-delivered siRNA and doxorubicin to mice via tail injection and were accumulated at the tumor site [
239]. Conversely, Jiang et al. proposed the use of cation-free self-assembled micelles based on siRNA conjugates linked to poly(
N-isopropylacrylamide) diblock copolymer via a redox-sensitive disulfide bond. These siRNA micelles displayed effective BBB penetration for the treatment of glioblastoma. Moreover, synergistic therapy was demonstrated in a temozolomide-resistant tumor when the chemotherapeutic drug temozolomide was co-delivered with siRNA micelles to knockdown tumor-associated genes [
45].
Although synthetic polymers dominate the field of PM-mediated gene delivery, various natural compounds such as chitosan [
240], hyaluronic acid [
241] and cyclodextrins [
242] are also explored for siRNA delivery. Among others, chitosan offers distinct advantages as a natural polymer to develop non-viral vectors for siRNA. Chitosan is a positively charged non-toxic, biocompatible and biodegradable natural polysaccharide [
243]. Self-assembling cholesterol-conjugated chitosan micelles co-delivering siRNA and salinomycin showed improved in vitro cytotoxicity against both SNU-668 and SGC-791 gastric cancer cells and more potent tumor suppression compared to free salinomycin in vivo [
244]. Recently, carboxymethyl chitosan, a water-soluble derivative of chitosan, was utilized to prepare multifunctional micelles grafted with an epidermal growth factor receptor (EGFR)-specific ligand, GE11 peptide, for tumor targeting. The co-delivery of doxorubicin with PD-L1 siRNA, which can inhibit PD-L1 expression and reactivate immune responses against malignant cells, using peptide-modified carboxymethyl chitosan micelles enhanced the anti-tumor effect in an orthotopic-tumor-bearing mouse model when administered i.v. [
245].
This entry is adapted from the peer-reviewed paper 10.3390/pharmaceutics15082021