1. Introduction
Clean water resources are under stress globally to meet growing demand. The rapid industrial and urban growths often cause water pollution [
1,
2]. Many industries in developing countries discharge untreated wastewater into surface water bodies [
3,
4]. Many primary water sources such as groundwater and surface water bodies in both developing and developed countries are exposed to various industrial and agricultural pollutants [
5,
6]. As a result, people suffer from cancer and other diseases, which are caused by water pollution among other factors [
7,
8,
9]. In many rural areas, groundwater is either unavailable or contaminated (such as arsenic and fluoride contamination). Currently, 783 million people do not have access to clean water, and 84% of them live in rural areas of developing countries. Many rural communities have been suffering from various acute waterborne diseases such as hepatitis, cholera, dysentery, giardiasis, diarrhoea, typhoid, and cryptosporidiosis [
10,
11,
12]. The water treatment cost is relatively high, and many developing countries cannot afford to treat polluted water [
13]. In many locations, rural and low-income communities are deprived of the benefit of modern water treatment technologies [
14].
To meet clean water demand, harvested rainwater (HRW) is getting more attention in remote and some urban areas as rainwater is generally fresh in nature [
15,
16,
17,
18,
19]. A question is often raised whether HRW needs disinfection before human consumption. For example, Chubbaka et al. [
20] identified that some households in South Australia prefer drinking untreated HRW; however, some residents treat HRW using a filtration system.
Research suggests that in many parts of the world, the quality of untreated HRW does not comply with the World Health Organisation’s (WHO) [
20,
21] drinking water guidelines. The reasons include (i) location of roof catchment [
22], (ii) surrounding sources of pollutants [
22], (iii) roof materials, (iv) air quality, (v) tank size, (vi) animal waste [
23], and (vii) materials used in HRW storage and collection systems [
24]. It indicates that at many instances HRW contains heavy metals [
25], and microbiological contaminants. The consumption of untreated HRW for an extended period may have both immediate and long-term health consequences [
21]. Therefore, HRW treatment at a small scale has become more relevant to the communities now than in the past.
Researchers indicate that the efficacy of water treatment methods may vary depending on the water source, environment, and catchment [
26]. For example, Brown and Sobsey [
27] conducted a test on the performance of ceramic filters between two types of water catchments, (a) rainwater and (b) surface water. They showed that rainwater has less turbidity compared to surface water. Lantagne et al. [
28] proposed five well-known methods for household water treatment: (i) chlorination, (ii) filtration (bio-sand and ceramic), (iii) solar disinfection, (iv) combination of filtration and chlorination, and (v) combination of flocculation and chlorination. They noted that water quality improved significantly after treatment, reducing water-borne diseases. They also compared the performances of various filtration and disinfection methods as shown in
Table 1.
Many non-governmentgovernment organisations (NGOs) have used hypochlorite solution for water disinfection at domestic level [
32,
33,
34] because hypochlorite solution has additional advantages such as (i) lower cost, (ii) residual effect, and (iii) greater availability [
34]. Among the advantages, the residual effect is one of the critical factors for hypo-chlorite solution, which can prevent stored water from potential recontamination. Ali et al. [
35] noticed regrowth of coliform bacteria after treating water by coagulation. Therefore, it can be argued that there is a potential risk of recontamination of the disinfected rainwater while it is being stored. Hence, a hypochlorite solution could be an effective and preferred disinfectant for rainwater disinfection.
However, using the hypochlorite solution has some concerns that must be considered before recommending it for disinfecting HRW at the household level. Hypochlorite solution can produce disinfection byproducts (DBP) when it reacts with organic contents present in water [
28,
36]. HRW may contain organic matter, and hypochlorite solution can produce DBPs when applied to untreated rainwater [
37]. DBP consumption through drinking water may trigger severe health issues like cancer [
38]. Hence, both inadequate and excess chlorine dose in water treatment is undesirable. Raising awareness on microbiological contaminants and chlorine DBPs in HRW is essential to people who consume rainwater.
2. Water Quality of Harvested Rainwater (HRW): Secondary Data Analysis of Microbiological Contaminants
Bacteria, viruses, and protozoa are the major microbiological pollutants in HRW [
39]. The primary sources of microbial contaminants are the faeces of animals such as birds, rats, squirrels, and possums [
23]. These unwanted animal excrements are washed out from the roof and accumulated in rainwater tanks during rainfall.
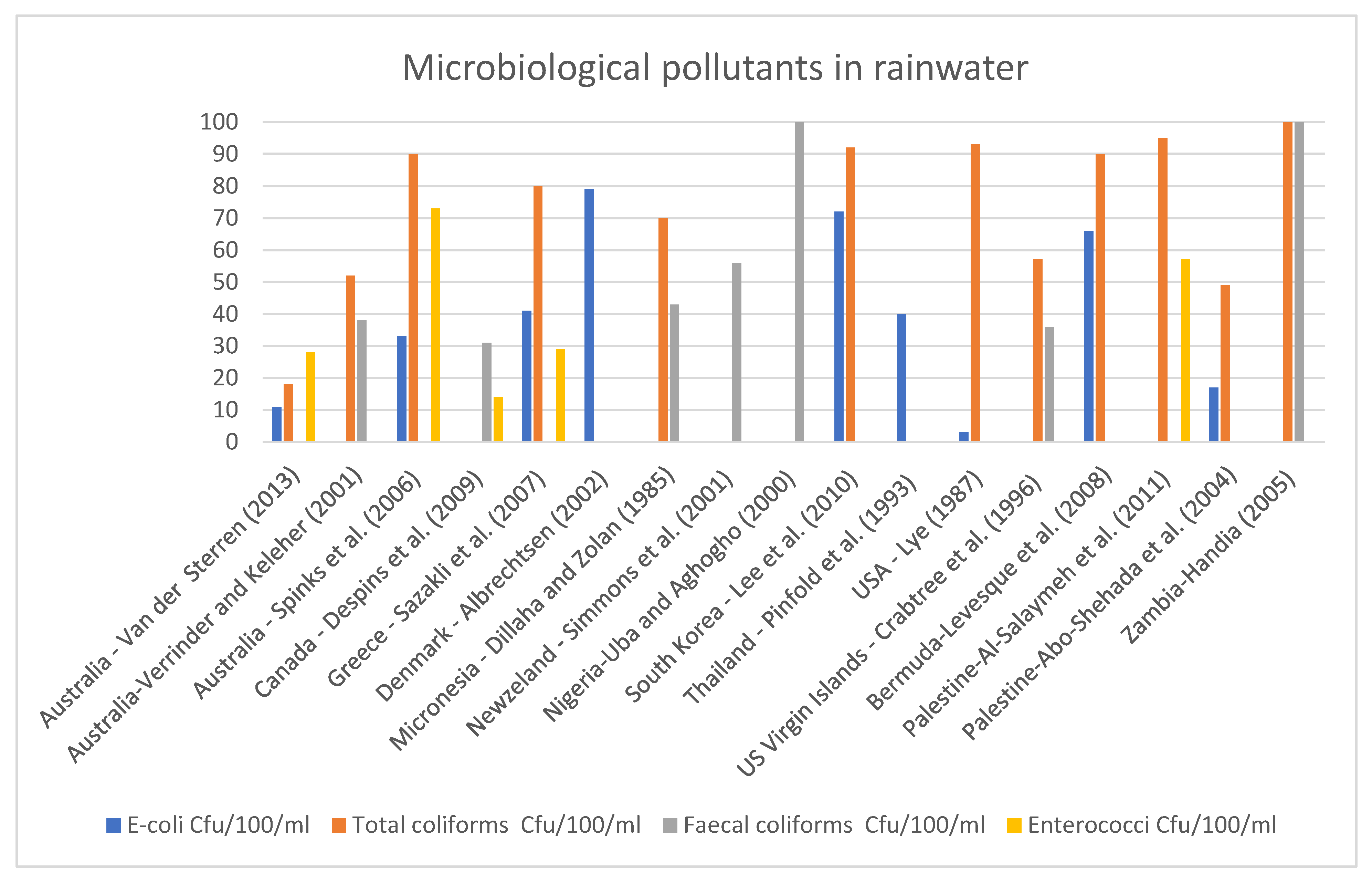
Figure 1 shows various harmful microorganisms in rainwater tank collected from 15 countries. Among the microbiological pollutants, faecal and total coliform have been identified in higher concentrations than
E. coli and Enterococci.
Figure 1 demonstrates that faecal and total coliform were found excessive in rainwater worldwide in both developed and developing countries. This indicates that rainwater treatment is mainly ignored on a small scale, irrespective of the country’s economic status.
Figure 1. Microbiological pollutants in harvested rainwater (HRW) in various countries [
40,
41,
42,
43,
44,
45,
46,
47,
48,
49,
50,
51,
52,
53,
54,
55,
56].
WHO drinking water guidelines [
57] suggest that drinking water must be free from
E. coli, total coliform, and thermotolerant coliform bacteria. However,
Figure 1 demonstrates that rainwater contains a substantial amount of these microbiological pollutants [
21]. They are responsible for waterborne diseases such as Diarrhoea, Cholera, Typhoid, and long-term gastrointestinal disorders [
58]. It was further noticed that less awareness and poor preventive control spread these diseases quickly across the community [
59].
Figure 2 further explains human health damage while consuming untreated HRW. Diarrhoea and vomiting are the most common symptoms when waterborne diseases infect the body.
Figure 2. Microorganisms that are responsible for waterborne diseases.
Furthermore, remote communities are more vulnerable as they experience delays in having primary care services than large cities [
60,
61]. Therefore, the most viable solution to avert the spreading of disease in remote areas is to treat the rainwater and ensure water is clean and disinfected [
59,
62]. Therefore, it can be argued that untreated rainwater is not recommended to drink.
3. Opportunities for Hypochlorite Solution to Disinfect HRW
Among the disinfection methods, chlorination is the most common method in large-scale water treatment schemes [
38,
63,
64,
65]. It is also one of the most cost-effective water disinfection processes [
66]. It is further suggested that free chlorine could be extensively efficacious against most waterborne pathogens and cause destruction of the cell DNA of these microorganisms, except for Cryptosporidium parvum oocysts and Mycobacteria species [
66]. Consequently, this method could reduce waterborne diseases in many countries [
32,
47]. In addition, chlorine tablets and liquid pool chlorine are reasonably accessible and safe for transportation to the end-users for residential purposes [
32].
Despite having some advantages, chlorination is not the preferred option in the residential or small-scale context [
67,
68]. Alekal et al. [
67] and Burch and Thomas [
29] identified three primary reasons which deter dwellers from implementing chlorination in their houses for water disinfection: (1) difficulties in mixing chemicals, (2) lack of convenience in storing chemicals, and (3) poor taste and odour issues because of incorrect dosing.
4. Chlorination for Disinfection
Various chlorine compounds are used for disinfection, such as chlorine gas, sodium-hypochlorite, chlorine tablets, calcium hypochlorite, bleaching powder, and chlorine dioxide [
69,
70]. The most common ones are (a) chlorine gas, (b) liquid pool chlorine (sodium hypochlorite, NaOCL) solution, and (c) calcium hypochlorite Ca(OCl)
2 as solid [
71]. When a chlorine-contained disinfectant is administered in water, it transforms into hypo-chlorous acid (HOCl) and hypochlorite ion (OCl
−). Free available chlorine (FAC) is the combination of HOCl and OCl
- in water [
70,
71]. The associated chemical reactions are shown in Equations (1)–(4):
Similarly, when NaOCL and Ca(OCl)
2 are applied, they also form HOCl:
Wei-Ling and Jensen [
70] suggested total residual chlorine combines FAC and chloramine concentrations in water. FAC predominantly reacts with ammonia in water and forms mono-chloramine first and then dichloramine [
70]. Also, FAC reacts with multiple substances available in water and influences chlorine demand [
70].
Amy et al. [
71] suggested hypochlorite ion is an active oxidising substance. Hypochlorite ion originated from the reversible ionisation process of (HOCl) as shown in Equation (2). However, HOCl performs better than OCl
− against germs [
71]. On the contrary, the unwanted microorganisms regrow in treated water after free chlorine fades out [
72,
73]. Amy et al. [
63] stated hypochlorous acid is more stable if pH remains under 7.5; otherwise, it turns to OCl
−. Amy et al. [
63] further suggested that chlorination’s breaking point occurs when the total residual concentration remains unchanged.
Wei-Ling and Jensen [
70] and Alim et al. [
72] conducted tests on chlorine decay in laboratories, and they added chlorine and hypochlorite to water sample, respectively. They identified that total residual chlorine (TRC) dropped initially and increased later when chlorination proceeded further. When the chlorine dose exceeds the TRC, it indicates the breakpoint. Alim et al. [
72] suggested that Sydney Water Corporation’s standard is to maintain chlorine levels between 1 and 3 mg/L. According to WHO [
73] guidelines, chlorine contact time (CT) should be held at 15 mg. min/L for proper disinfection. That means the free chlorine residual must be kept more than 0.5 mg/L in the water for 30 min.
This entry is adapted from the peer-reviewed paper 10.3390/w15152816