Spinal cord injury (SCI) is a catastrophic condition associated with significant neurological deficit and social and financial burdens. The advancements in biomaterial technology, combined with stem cell therapy or other regenerative therapy, can now accelerate the progress of promising novel therapeutic strategies from bench to bedside. Various types of approaches to regeneration therapy for SCI have been combined with the use of supportive biomaterial scaffolds as a drug and cell delivery system to facilitate favorable cell–material interactions and the supportive effect of neuroprotection.
- biomaterial
- combination therapy
- regenerative medicine
- scaffold
- spinal cord injury
1. Introduction
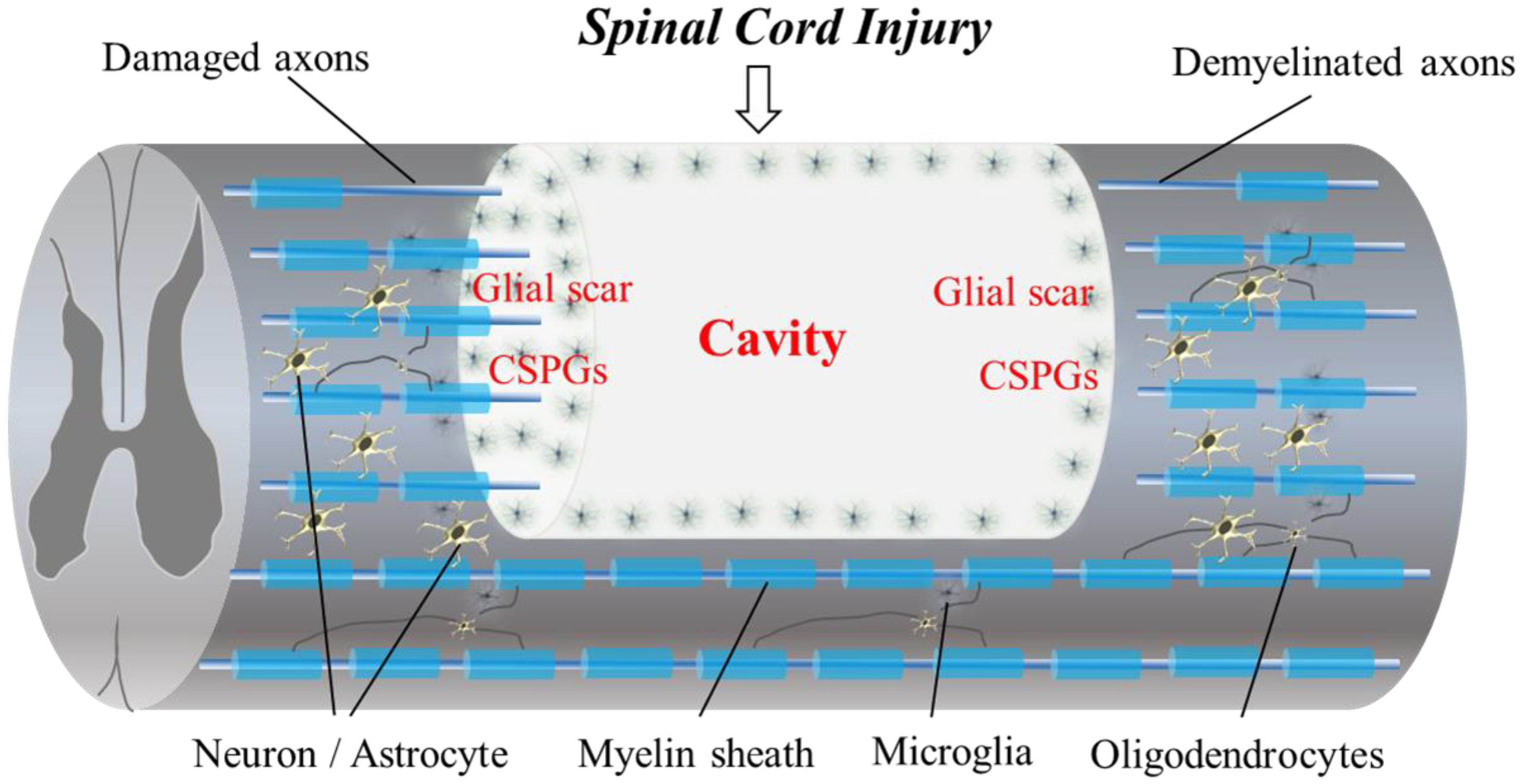
2. Barriers to Regeneration and the Pathophysiology of SCI
3. Categories of Biomaterial Scaffolds Applied in Regeneration Therapy for SCI
3.1. Hydrogels
3.2. Biodegradable Scaffolds
3.3. Nano- and Micro-Scale Scaffolds as Instructive Biomaterials for SCI
4. Biomaterial Scaffolds in Combinatory Treatment Used for DDSs in SCI Treatment
| Effect on Pathophysiological Events | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Author, Year | Location of Injury | Species | Combinatory Agent | Biomaterial Scaffold | Anti-Inflammation | Scar/Cavity | Axon Growth | Angiogenesis | Facilitation of Cell Migration | Motor Functional Recovery |
| Furuya T, et al., 2013 [105] |
Thoracic | Rat | bFGF | Gelatin hydrogel | NA | NA | NA | NA | NA | NA |
| Chantal SA, et al., 2008 [106] |
Thoracic | Rat | Methylprednisolone | Biodegradable PLGA-based nanoparticles | + | + | NA | NA | + | NA |
| Jain A, et al., 2011 [107] |
Thoracic | Rat | Constitutively active Cdc42, Rac1, BDNF |
Microtubule-mediated slow release of BDNF | + | + | + | NA | + | NA |
| Wen Y et al., 2016 [108] |
Thoracic | Rat | Anti-Nogo receptor antibody | PLGA microspheres containing BDNF and VEGF |
+ | + | + | + | + | + |
| Chen B, et al., 2015 [109] |
Thoracic | Rat | bFGF | HEMA-MOETACL hydrogel | NA | + | + | NA | NA | + |
| Lin J, et al., 2019 [110] |
Thoracic | Rat | Rehabilitation | Hybrid fiber-hydrogel scaffold | + | + | + | NA | + | + |
| Shi Q, et al., 2014 [111] |
Thoracic | Rat | bFGF | Collagen scaffold | NA | + | + | NA | + | + |
| Wang X, et al., 2013 [112] |
Thoracic | Rat | NT-3 | Chitosan-based tube scaffold | NA | + | + | NA | + | + |
| Li G, et al., 2016 [113] |
Thoracic | Rat and canine | NT-3 | Fibrin-coated gelatin sponge scaffold | + | + | + | NA | + | + |
| Wei YT, et al., 2010 [114] |
Thoracic | Rat | Nogo-66 receptor antibody | Hyaluronic acid -based hydrogels modified with poly-L-lysine (PLL) | + | + | + | + | + | NA |
| Bighinati A, et al., 2020 [115] |
Thoracic | Rat | Ibuprofen and triiodothyronine | PLLA | + | + | + | NA | + | + |
| Ehsanipour A, et al., 2021 [116] |
Thoracic | Mouse | BDNF | Hyaluronic acid (HA)-based, spherical microparticle | + | + | + | NA | + | + |
| Xie J, et al., 2022 [117] |
Thoracic | Mouse | Sonic hedgehog (Shh) and retinoic acid (RA) |
Magnesium oxide (MgO)/ poly (l-lactide-co-ε-caprolactone) (PLCL) scaffold |
+ | + | + | NA | + | NA |
| Xi K, et al., 2020 [118] |
Thoracic | Rat | NGF | Microenvironment-responsive immunoregulatory electrospun fibers |
+ | + | + | NA | + | + |
| Rooney GE, et al., 2011 [119] |
Thoracic | Rat | Dibutyryl cyclic adenosine monophosphate (dbcAMP) | Oligo [(polyethylene glycol) fumarate] (OPF) hydrogel scaffolds | NA | NA | + | NA | NA | NA |
| Stropkovská A, et al., 2022 [120] |
Thoracic | Rat | Rho-A-kinase inhibitor | Chitosan/collagen porous scaffold | + | + | + | NA | + | NA |
| Man W, et al., 2021 [72] |
Thoracic | Rat | Hierarchically aligned fibrin hydrogel | Functionalized self-assembling peptides (fSAP) |
+ | + | + | + | + | + |
| Smith DR, et al., 2020 [121] |
Cervical | Mouse | IL-10 and NT-3 | Multiple channel PLG | + | NA | + | NA | + | + |
| Breen BA, et al., 2017 [122] |
Thoracic | Rat | NT-3 | Injectable collagen scaffold | NA | + | + | NA | + | + |
| Wen Y et al., 2016 [108] |
Thoracic | Rat | AntiNogo, BDNF and vascular endothelial growth factor |
Hyaluronic acid (HA) hydrogel | + | + | + | + | + | + |
| Jain A, et al., 2006 [123] |
Thoracic | Rat | BDNF | Gelling agarose hydrogels | NA | + | + | NA | + | NA |
This entry is adapted from the peer-reviewed paper 10.3390/ijms24032528
References
- Fehlings, M.G.; Martin, A.R.; Tetreault, L.A.; Aarabi, B.; Anderson, P.; Arnold, P.M.; Brodke, D.; Burns, A.; Chiba, K.; Dettori, J.R.; et al. A clinical practice guideline for the management of patients with acute spinal cord injury: Recommendations on the role of baseline magnetic resonance imaging in clinical decision making and outcome prediction. Glob. Spine J. 2017, 7, 221S–230S.
- Fehlings, M.G.; Kim, K.D.; Aarabi, B.; Rizzo, M.; Bond, L.M.; McKerracher, L.; Vaccaro, A.R.; Okonkwo, D.O. Rho Inhibitor VX-210 in Acute Traumatic Subaxial Cervical Spinal Cord Injury: Design of the SPinal Cord Injury Rho INhibition InvestiGation (SPRING) Clinical Trial. J. Neurotrauma 2018, 35, 1049–1056.
- Singh, A.; Tetreault, L.; Kalsi-Ryan, S.; Nouri, A.; Fehlings, M.G. Global prevalence and incidence of traumatic spinal cord injury. Clin. Epidemiol. 2014, 6, 309–331.
- Spinal Cord Injury (SCI) 2016 Facts and Figures at a Glance. J. Spinal Cord Med. 2016, 39, 493–494.
- Costăchescu, B.; Niculescu, A.-G.; Dabija, M.G.; Teleanu, R.I.; Grumezescu, A.M.; Eva, L. Novel Strategies for Spinal Cord Regeneration. Int. J. Mol. Sci. 2022, 23, 4552.
- Ahuja, C.S.; Fehlings, M. Concise Review: Bridging the Gap: Novel Neuroregenerative and Neuroprotective Strategies in Spinal Cord Injury. Stem Cells Transl. Med. 2016, 5, 914–924.
- Zweckberger, K.; Ahuja, C.S.; Liu, Y.; Wang, J.; Fehlings, M.G. Self-assembling peptides optimize the post-traumatic milieu and synergistically enhance the effects of neural stem cell therapy after cervical spinal cord injury. Acta Biomater. 2016, 42, 77–89.
- Kawabata, S.; Takano, M.; Numasawa-Kuroiwa, Y.; Itakura, G.; Kobayashi, Y.; Nishiyama, Y.; Sugai, K.; Nishimura, S.; Iwai, H.; Isoda, M.; et al. Grafted Human iPS Cell-Derived Oligodendrocyte Precursor Cells Contribute to Robust Remyelination of Demyelinated Axons after Spinal Cord Injury. Stem Cell Rep. 2016, 6, 1–8.
- Lu, P.; Kadoya, K.; Tuszynski, M.H. Axonal growth and connectivity from neural stem cell grafts in models of spinal cord injury. Curr. Opin. Neurobiol. 2014, 27, 103–109.
- Lis, A.; Szarek, D.; Laska, J. Strategie inzynierii biomateriałów dla regeneracji rdzenia kregowego: Aktualny stan wiedzy. Polym. Med. 2013, 43, 59–80.
- Imagama, T.; Ogino, K.; Takemoto, K.; Kato, Y.; Kataoka, H.; Suzuki, H.; Ran, Z.; Setiawan, H.; Fujikura, Y.; Taguchi, T. Regulation of nitric oxide generation by up-regulated arginase I in rat spinal cord injury. J. Clin. Biochem. Nutr. 2012, 51, 68–75.
- Murakami, T.; Kanchiku, T.; Suzuki, H.; Imajo, Y.; Yoshida, Y.; Nomura, H.; Cui, D.; Ishikawa, T.; Ikeda, E.; Taguchi, T. Anti-interleukin-6 receptor antibody reduces neuropathic pain following spinal cord injury in mice. Exp. Ther. Med. 2013, 6, 1194–1198.
- Suzuki, H.; Taguchi, T.; Kato, Y.; Kanchiku, T.; Imagama, T.; Yara, T.; Moriya, A.; Muramatsu, K.; Tanaka, H.; Gondo, T. Transplantation of neurospheres derived from bone marrow stromal cells promotes neurological recovery in rats with spinal cord injury. Med. Mol. Morphol. 2011, 44, 131–138.
- Wilcox, J.T.; Satkunendrarajah, K.; Zuccato, J.A.; Nassiri, F.; Fehlings, M.G. Neural Precursor Cell Transplantation Enhances Functional Recovery and Reduces Astrogliosis in Bilateral Compressive/Contusive Cervical Spinal Cord Injury. Stem Cells Transl. Med. 2014, 3, 1148–1159.
- Suzuki, H.; Ahuja, C.S.; Salewski, R.P.; Li, L.; Satkunendrarajah, K.; Nagoshi, N.; Shibata, S.; Fehlings, M.G. Neural stem cell mediated recovery is enhanced by Chondroitinase ABC pretreatment in chronic cervical spinal cord injury. PLoS ONE 2017, 12, e0182339.
- Ramon y Cajal, S. Degeneration and Regeneration of the Nervous System; Oxford University Press: London, UK, 1928.
- Bradbury, E.J.; Moon, L.D.F.; Popat, R.J.; King, V.R.; Bennett, G.S.; Patel, P.N.; Fawcett, J.W.; McMahon, S.B. Chondroitinase ABC promotes functional recovery after spinal cord injury. Nature 2002, 416, 636–640.
- Suzuki, H.; Imajo, Y.; Funaba, M.; Nishida, N.; Sakamoto, T.; Sakai, T. Current Concepts of Neural Stem/Progenitor Cell Therapy for Chronic Spinal Cord Injury. Front. Cell. Neurosci. 2022, 15, 794692.
- Suzuki, H.; Sakai, T. Current Concepts of Stem Cell Therapy for Chronic Spinal Cord Injury. Int. J. Mol. Sci. 2021, 22, 7435.
- Ahuja, C.S.; Nori, S.; Tetreault, L.; Wilson, J.; Kwon, B.; Harrop, J.; Choi, D.; Fehlings, M.G. Traumatic Spinal Cord Injury—Repair and Regeneration. Neurosurgery 2017, 80, S9–S22.
- Cofano, F.; Boido, M.; Monticelli, M.; Zenga, F.; Ducati, A.; Vercelli, A.; Garbossa, D. Mesenchymal Stem Cells for Spinal Cord Injury: Current Options Limitations, and Future of Cell Therapy. Int. J. Mol. Sci. 2019, 20, 2698.
- Hutson, T.H.; Di Giovanni, S. The translational landscape in spinal cord injury: Focus on neuroplasticity and regeneration. Nat. Rev. Neurol. 2019, 15, 732–745.
- Badhiwala, J.H.; Wilson, J.R.; Kwon, B.K.; Casha, S.; Fehlings, M.G. A Review of Clinical Trials in Spinal Cord Injury Including Biomarkers. J. Neurotrauma 2018, 35, 1906–1917.
- Shinozaki, M.; Nagoshi, N.; Nakamura, M.; Okano, H. Mechanisms of Stem Cell Therapy in Spinal Cord Injuries. Cells 2021, 10, 2676.
- Ahuja, C.S.; Mothe, A.; Khazaei, M.; Badhiwala, J.H.; Gilbert, E.A.; van der Kooy, D.; Morshead, C.M.; Tator, C.; Fehlings, M.G. The leading edge: Emerging neuroprotective and neuroregenerative cell-based therapies for spinal cord injury. Stem Cells Transl. Med. 2020, 9, 1509–1530.
- Gabel, B.C.; Curtis, E.I.; Marsala, M.; Ciacci, J.D. A Review of Stem Cell Therapy for Spinal Cord Injury: Large Animal Models and the Frontier in Humans. World Neurosurg. 2017, 98, 438–443.
- Zipser, C.M.; Cragg, J.J.; Guest, J.D.; Fehlings, M.G.; Jutzeler, C.R.; Anderson, A.J.; Curt, A. Cell-based and stem-cell-based treatments for spinal cord injury: Evidence from clinical trials. Lancet Neurol. 2022, 21, 659–670.
- Kiyotake, E.A.; Martin, M.D.; Detamore, M.S. Regenerative rehabilitation with conductive biomaterials for spinal cord injury. Acta Biomater. 2022, 139, 43–64.
- Ghane, N.; Beigi, M.-H.; Labbaf, S.; Nasr-Esfahani, M.-H.; Kiani, A. Design of hydrogel-based scaffolds for the treatment of spinal cord injuries. J. Mater. Chem. B 2020, 8, 10712–10738.
- Pina, S.; Ribeiro, V.P.; Marques, C.F.; Maia, F.R.; Silva, T.H.; Reis, R.L.; Oliveira, J.M. Scaffolding Strategies for Tissue Engineering and Regenerative Medicine Applications. Materials 2019, 12, 1824.
- Estrada, V.; Tekinay, A.; Müller, H.W. Neural ECM mimetics. Prog. Brain Res. 2014, 214, 391–413.
- Khaing, Z.Z.; Seidlits, S.K. Hyaluronic acid and neural stem cells: Implications for biomaterial design. J. Mater. Chem. B 2015, 3, 7850–7866.
- Sun, Y.; Yang, C.; Zhu, X.; Wang, J.-J.; Liu, X.-Y.; Yang, X.-P.; An, X.-W.; Liang, J.; Dong, H.-J.; Jiang, W.; et al. 3D printing collagen/chitosan scaffold ameliorated axon regeneration and neurological recovery after spinal cord injury. J. Biomed. Mater. Res. Part A 2019, 107, 1898–1908.
- Marchand, R.; Woerly, S. Transected spinal cords grafted with in situ self-assembled collagen matrices. Neuroscience 1990, 36, 45–60.
- Khan, T.; Dauzvardis, M.; Sayers, S. Carbon filament implants promote axonal growth across the transected rat spinal cord. Brain Res. 1991, 541, 139–145, Erratum in Brain Res. 1991, 546, 360.
- Liu, W.; Xu, B.; Xue, W.; Yang, B.; Fan, Y.; Chen, B.; Xiao, Z.; Xue, X.; Sun, Z.; Shu, M.; et al. A functional scaffold to promote the migration and neuronal differentiation of neural stem/progenitor cells for spinal cord injury repair. Biomaterials 2020, 243, 119941.
- Fan, C.; Li, X.; Xiao, Z.; Zhao, Y.; Liang, H.; Wang, B.; Han, S.; Li, X.; Xu, B.; Wang, N.; et al. A modified collagen scaffold facilitates endogenous neurogenesis for acute spinal cord injury repair. Acta Biomater. 2017, 51, 304–316.
- Yang, B.; Liang, C.; Chen, D.; Cheng, F.; Zhang, Y.; Wang, S.; Shu, J.; Huang, X.; Wang, J.; Xia, K.; et al. A conductive supramolecular hydrogel creates ideal endogenous niches to promote spinal cord injury repair. Bioact. Mater. 2022, 15, 103–119.
- Martín-López, E.; Darder, M.; Ruiz-Hitzky, E.; Sampedro, M.N. Agar-based bridges as biocompatible candidates to provide guide cues in spinal cord injury repair. Bio-Med. Mater. Eng. 2013, 23, 405–421.
- Gros, T.; Sakamoto, J.S.; Blesch, A.; Havton, L.A.; Tuszynski, M.H. Regeneration of long-tract axons through sites of spinal cord injury using templated agarose scaffolds. Biomaterials 2010, 31, 6719–6729.
- Kataoka, K.; Suzuki, Y.; Kitada, M.; Hashimoto, T.; Chou, H.; Bai, H.; Ohta, M.; Wu, S.; Suzuki, K.; Ide, C. Alginate Enhances Elongation of Early Regenerating Axons in Spinal Cord of Young Rats. Tissue Eng. 2004, 10, 493–504.
- Prang, P.; Mueller, R.; Eljaouhari, A.; Heckmann, K.; Kunz, W.; Weber, T.; Faber, C.; Vroemen, M.; Bogdahn, U.; Weidner, N. The promotion of oriented axonal regrowth in the injured spinal cord by alginate-based anisotropic capillary hydrogels. Biomaterials 2006, 27, 3560–3569.
- Cao, Z.; Yao, S.; Xiong, Y.; Zhang, Z.; Yang, Y.; He, F.; Zhao, H.; Guo, Y.; Wang, G.; Xie, S.; et al. Directional axonal regrowth induced by an aligned fibrin nanofiber hydrogel contributes to improved motor function recovery in canine L2 spinal cord injury. J. Mater. Sci. Mater. Med. 2020, 31, 40.
- Yin, W.; Xue, W.; Zhu, H.; Shen, H.; Xiao, Z.; Wu, S.; Zhao, Y.; Cao, Y.; Tan, J.; Li, J.; et al. Scar tissue removal-activated endogenous neural stem cells aid Taxol-modified collagen scaffolds in repairing chronic long-distance transected spinal cord injury. Biomater. Sci. 2021, 9, 4778–4792.
- Altinova, H.; Hammes, S.; Palm, M.; Achenbach, P.; Gerardo-Nava, J.; Deumens, R.; Führmann, T.; van Neerven, S.G.; Hermans, E.; Weis, J.; et al. Dense fibroadhesive scarring and poor blood vessel-maturation hamper the integration of implanted collagen scaffolds in an experimental model of spinal cord injury. Biomed. Mater. 2020, 15, 015012.
- Gholami, M.; Gilanpour, H.; Sadeghinezhad, J.; Asghari, A. Facile fabrication of an erythropoietin-alginate/chitosan hydrogel and evaluation of its local therapeutic effects on spinal cord injury in rats. DARU J. Pharm. Sci. 2021, 29, 255–265.
- Stokols, S.; Tuszynski, M.H. Freeze-dried agarose scaffolds with uniaxial channels stimulate and guide linear axonal growth following spinal cord injury. Biomaterials 2006, 27, 443–451.
- Zhang, Z.; Yao, S.; Xie, S.; Wang, X.; Chang, F.; Luo, J.; Wang, J.; Fu, J. Effect of hierarchically aligned fibrin hydrogel in regeneration of spinal cord injury demonstrated by tractography: A pilot study. Sci. Rep. 2017, 7, 40017.
- Fukushima, K.; Enomoto, M.; Tomizawa, S.; Takahashi, M.; Wakabayashi, Y.; Itoh, S.; Kuboki, Y.; Shinomiya, K. The axonal regeneration across a honeycomb collagen sponge applied to the transected spinal cord. J. Med. Dent. Sci. 2008, 55, 71–79.
- Zhao, X.; Wang, H.; Zou, Y.; Xue, W.; Zhuang, Y.; Gu, R.; Shen, H.; Dai, J. Optimized, visible light-induced crosslinkable hybrid gelatin/hyaluronic acid scaffold promotes complete spinal cord injury repair. Biomed. Mater. 2022, 17, 024104.
- King, V.R.; Alovskaya, A.; Wei, D.; Brown, R.A.; Priestley, J.V. The use of injectable forms of fibrin and fibronectin to support axonal ingrowth after spinal cord injury. Biomaterials 2010, 31, 4447–4456.
- Cheng, H.; Huang, Y.-C.; Chang, P.-T.; Huang, Y.-Y. Laminin-incorporated nerve conduits made by plasma treatment for repairing spinal cord injury. Biochem. Biophys. Res. Commun. 2007, 357, 938–944.
- Han, S.; Lee, J.Y.; Heo, E.Y.; Kwon, I.K.; Yune, T.Y.; Youn, I. Implantation of a Matrigel-loaded agarose scaffold promotes functional regeneration of axons after spinal cord injury in rat. Biochem. Biophys. Res. Commun. 2018, 496, 785–791.
- Bakshi, A.; Fisher, O.; Dagci, T.; Himes, B.T.; Fischer, I.; Lowman, A. Mechanically engineered hydrogel scaffolds for axonal growth and angiogenesis after transplantation in spinal cord injury. J. Neurosurg. Spine 2004, 1, 322–329.
- Zhai, H.; Zhou, J.; Xu, J.; Sun, X.; Xu, Y.; Qiu, X.; Zhang, C.; Wu, Z.; Long, H.; Bai, Y.; et al. Mechanically strengthened hybrid peptide-polyester hydrogel and potential applications in spinal cord injury repair. Biomed. Mater. 2020, 15, 055031.
- Hejčl, A.; Růžička, J.; Kekulová, K.; Svobodová, B.; Proks, V.; Macková, H.; Jiránková, K.; Kárová, K.; Urdziková, L.M.; Kubinová, Š.; et al. Modified Methacrylate Hydrogels Improve Tissue Repair after Spinal Cord Injury. Int. J. Mol. Sci. 2018, 19, 2481.
- Zhang, Q.; Yan, S.; You, R.; Kaplan, D.L.; Liu, Y.; Qu, J.; Li, X.; Li, M.; Wang, X. Multichannel silk protein/laminin grafts for spinal cord injury repair. J. Biomed. Mater. Res. Part A 2016, 104, 3045–3057.
- Chai, Y.; Long, Y.; Dong, X.; Liu, K.; Wei, W.; Chen, Y.; Qiu, T.; Dai, H. Improved functional recovery of rat transected spinal cord by peptide-grafted PNIPAM based hydrogel. Colloids Surf. B Biointerfaces 2022, 210, 112220.
- Silva, N.A.; Salgado, A.J.; Sousa, R.A.; Oliveira, J.T.; Pedro, A.J.; Leite-Almeida, H.; Cerqueira, R.; Almeida, A.; Mastronardi, F.; Mano, J.F.; et al. Development and Characterization of a Novel Hybrid Tissue Engineering–Based Scaffold for Spinal Cord Injury Repair. Tissue Eng. Part A 2010, 16, 45–54.
- Yang, Y.; Fan, Y.; Zhang, H.; Zhang, Q.; Zhao, Y.; Xiao, Z.; Liu, W.; Chen, B.; Gao, L.; Sun, Z.; et al. Small molecules combined with collagen hydrogel direct neurogenesis and migration of neural stem cells after spinal cord injury. Biomaterials 2021, 269, 120479.
- Suzuki, H.; Kanchiku, T.; Imajo, Y.; Yoshida, Y.; Nishida, N.; Gondo, T.; Yoshii, S.; Taguchi, T. Artificial collagen-filament scaffold promotes axon regeneration and long tract reconstruction in a rat model of spinal cord transection. Med. Mol. Morphol. 2015, 48, 214–224.
- Yara, T.; Kato, Y.; Kataoka, H.; Kanchiku, T.; Suzuki, H.; Gondo, T.; Yoshii, S.; Taguchi, T. Environmental factors involved in axonal regeneration following spinal cord transection in rats. Med. Mol. Morphol. 2009, 42, 150–154.
- Nair, L.S.; Laurencin, C.T. Biodegradable polymers as biomaterials. Prog. Polym. Sci. 2007, 32, 762–798.
- Cheng, Y.; Zhang, Y.; Wu, H. Polymeric Fibers as Scaffolds for Spinal Cord Injury: A Systematic Review. Front. Bioeng. Biotechnol. 2022, 9, 807533.
- Iwasaki, M.; Wilcox, J.T.; Nishimura, Y.; Zweckberger, K.; Suzuki, H.; Wang, J.; Liu, Y.; Karadimas, S.K.; Fehlings, M.G. Synergistic effects of self-assembling peptide and neural stem/progenitor cells to promote tissue repair and forelimb functional recovery in cervical spinal cord injury. Biomaterials 2014, 35, 2617–2629.
- Kubinová, Š.; Horák, D.; Hejčl, A.; Plichta, Z.; Kotek, J.; Proks, V.; Forostyak, S.; Syková, E. SIKVAV-modified highly superporous PHEMA scaffolds with oriented pores for spinal cord injury repair. J. Tissue Eng. Regen. Med. 2015, 9, 1298–1309.
- Hejčl, A.; Urdzikova, L.M.; Sedy, J.; Lesny, P.; Pradny, M.; Michálek, J.; Burian, M.; Hajek, M.; Zamecnik, J.; Jendelova, P.; et al. Acute and delayed implantation of positively charged 2-hydroxyethyl methacrylate scaffolds in spinal cord injury in the rat. J. Neurosurg. Spine 2008, 8, 67–73.
- Sun, F.; Shi, T.; Zhou, T.; Dong, D.; Xie, J.; Wang, R.; An, X.; Chen, M.; Cai, J. 3D Poly(Lactic-co-glycolic acid) Scaffolds for Treating Spinal Cord Injury. J. Biomed. Nanotechnol. 2017, 13, 290–302.
- Slotkin, J.R.; Pritchard, C.D.; Luque, B.; Ye, J.; Layer, R.T.; Lawrence, M.S.; O’Shea, T.M.; Roy, R.R.; Zhong, H.; Vollenweider, I.; et al. Biodegradable scaffolds promote tissue remodeling and functional improvement in non-human primates with acute spinal cord injury. Biomaterials 2017, 123, 63–76.
- Silva, N.A.; Sousa, R.A.; Fraga, J.S.; Fontes, M.; Leite-Almeida, H.; Cerqueira, R.; Almeida, A.; Sousa, N.; Reis, R.L.; Salgado, A.J. Benefits of Spine Stabilization with Biodegradable Scaffolds in Spinal Cord Injured Rats. Tissue Eng. Part C Methods 2013, 19, 101–108.
- Thomas, A.M.; Kubilius, M.B.; Holland, S.J.; Seidlits, S.K.; Boehler, R.M.; Anderson, A.J.; Cummings, B.J.; Shea, L.D. Channel density and porosity of degradable bridging scaffolds on axon growth after spinal injury. Biomaterials 2013, 34, 2213–2220.
- Man, W.; Yang, S.; Cao, Z.; Lu, J.; Kong, X.; Sun, X.; Zhao, L.; Guo, Y.; Yao, S.; Wang, G.; et al. A multi-modal delivery strategy for spinal cord regeneration using a composite hydrogel presenting biophysical and biochemical cues synergistically. Biomaterials 2021, 276, 120971.
- Kubinová, Š.; Horák, D.; Hejčl, A.; Plichta, Z.; Kotek, J.; Syková, E. Highly superporous cholesterol-modified poly(2-hydroxyethyl methacrylate) scaffolds for spinal cord injury repair. J. Biomed. Mater. Res. Part A 2011, 99A, 618–629.
- Guest, J.D.; Moore, S.W.; Aimetti, A.A.; Kutikov, A.B.; Santamaria, A.J.; Hofstetter, C.P.; Ropper, A.E.; Theodore, N.; Ulich, T.R.; Layer, R.T. Internal decompression of the acutely contused spinal cord: Differential effects of irrigation only versus biodegradable scaffold implantation. Biomaterials 2018, 185, 284–300.
- Hakim, J.S.; Rodysill, B.R.; Chen, B.K.; Schmeichel, A.M.; Yaszemski, M.J.; Windebank, A.J.; Madigan, N.N. Combinatorial tissue engineering partially restores function after spinal cord injury. J. Tissue Eng. Regen. Med. 2019, 13, 857–873.
- Anzalone, A.; Chacko, J.V.; Nishi, R.A.; Dumont, C.; Smith, D.; Shea, L.D.; Digman, M.A.; Cummings, B.J.; Anderson, A.J. Feasibility study on mouse live imaging after spinal cord injury and poly(lactide-co-glycolide) bridge implantation. J. Biomed. Opt. 2018, 23, 065007.
- De Laporte, L.; Yan, A.L.; Shea, L.D. Local gene delivery from ECM-coated poly(lactide-co-glycolide) multiple channel bridges after spinal cord injury. Biomaterials 2009, 30, 2361–2368.
- Wong, D.Y.; Leveque, J.-C.; Brumblay, H.; Krebsbach, P.H.; Hollister, S.J.; LaMarca, F. Macro-Architectures in Spinal Cord Scaffold Implants Influence Regeneration. J. Neurotrauma 2008, 25, 1027–1037.
- Ribeiro-Samy, S.; Silva, N.A.; Correlo, V.M.; Fraga, J.S.; Pinto, L.; Teixeira-Castro, A.; Leite-Almeida, H.; Almeida, A.; Gimble, J.M.; Sousa, N.; et al. Development and Characterization of a PHB-HV-based 3D Scaffold for a Tissue Engineering and Cell-therapy Combinatorial Approach for Spinal Cord Injury Regeneration. Macromol. Biosci. 2013, 13, 1576–1592.
- Pawar, K.; Cummings, B.J.; Thomas, A.; Shea, L.D.; Levine, A.; Pfaff, S.; Anderson, A.J. Biomaterial bridges enable regeneration and re-entry of corticospinal tract axons into the caudal spinal cord after SCI: Association with recovery of forelimb function. Biomaterials 2015, 65, 1–12.
- Rooney, G.E.; Vaishya, S.; Ameenuddin, S.; Currier, B.L.; Schiefer, T.K.; Knight, A.; Chen, B.; Mishra, P.K.; Spinner, R.J.; Macura, S.I.; et al. Rigid Fixation of the Spinal Column Improves Scaffold Alignment and Prevents Scoliosis in the Transected Rat Spinal Cord. Spine 2008, 33, E914–E919.
- Shu, B.; Sun, X.; Liu, R.; Jiang, F.; Yu, H.; Xu, N.; An, Y. Restoring electrical connection using a conductive biomaterial provides a new therapeutic strategy for rats with spinal cord injury. Neurosci. Lett. 2019, 692, 33–40.
- Zhou, L.; Fan, L.; Yi, X.; Zhou, Z.; Liu, C.; Fu, R.; Dai, C.; Wang, Z.; Chen, X.; Yu, P.; et al. Soft Conducting Polymer Hydrogels Cross-Linked and Doped by Tannic Acid for Spinal Cord Injury Repair. ACS Nano 2018, 12, 10957–10967.
- Pertici, V.; Trimaille, T.; Laurin, J.; Felix, M.-S.; Marqueste, T.; Pettmann, B.; Chauvin, J.-P.; Gigmes, D.; Decherchi, P. Repair of the injured spinal cord by implantation of a synthetic degradable block copolymer in rat. Biomaterials 2014, 35, 6248–6258.
- Reis, K.P.; Sperling, L.E.; Teixeira, C.; Sommer, L.; Colombo, M.; Koester, L.S.; Pranke, P. VPA/PLGA microfibers produced by coaxial electrospinning for the treatment of central nervous system injury. Braz. J. Med. Biol. Res. 2020, 53, e8993.
- Novikova, L.N.; Kolar, M.K.; Kingham, P.J.; Ullrich, A.; Oberhoffner, S.; Renardy, M.; Doser, M.; Müller, E.; Wiberg, M.; Novikov, L.N. Trimethylene carbonate-caprolactone conduit with poly-p-dioxanone microfilaments to promote regeneration after spinal cord injury. Acta Biomater. 2018, 66, 177–191.
- Song, Y.H.; Agrawal, N.K.; Griffin, J.M.; Schmidt, C.E. Recent advances in nanotherapeutic strategies for spinal cord injury repair. Adv. Drug Deliv. Rev. 2019, 148, 38–59.
- Gerardo-Nava, J.; Führmann, T.; Klinkhammer, K.; Seiler, N.; Mey, J.; Klee, D.; Möller, M.; Dalton, P.D.; Brook, G.A. Human neural cell interactions with orientated electrospun nanofibers in vitro. Nanomedicine 2009, 4, 11–30.
- Zamani, F.; Amani-Tehran, M.; Latifi, M.; Shokrgozar, M.A.; Zaminy, A. Promotion of spinal cord axon regeneration by 3D nanofibrous core-sheath scaffolds. J. Biomed. Mater. Res. Part A 2014, 102, 506–513.
- Sun, X.; Bai, Y.; Zhai, H.; Liu, S.; Zhang, C.; Xu, Y.; Zou, J.; Wang, T.; Chen, S.; Zhu, Q.; et al. Devising micro/nano-architectures in multi-channel nerve conduits towards a pro-regenerative matrix for the repair of spinal cord injury. Acta Biomater. 2019, 86, 194–206.
- Cigognini, D.; Silva, D.; Paloppi, S.; Gelain, F. Evaluation of mechanical properties and therapeutic effect of injectable self-assembling hydrogels for spinal cord injury. J. Biomed. Nanotechnol. 2014, 10, 309–323.
- Yao, S.; Yu, S.; Cao, Z.; Yang, Y.; Yu, X.; Mao, H.-Q.; Wang, L.-N.; Sun, X.; Zhao, L.; Wang, X. Hierarchically aligned fibrin nanofiber hydrogel accelerated axonal regrowth and locomotor function recovery in rat spinal cord injury. Int. J. Nanomed. 2018, 13, 2883–2895.
- Altinova, H.; Möllers, S.; Deumens, R.; Gerardo-Nava, J.; Führmann, T.; van Neerven, S.G.A.; Bozkurt, A.; Mueller, C.A.; Hoff, H.J.; Heschel, I.; et al. Functional recovery not correlated with axon regeneration through olfactory ensheathing cell-seeded scaffolds in a model of acute spinal cord injury. Tissue Eng. Regen. Med. 2016, 13, 585–600.
- Usmani, S.; Biagioni, A.F.; Medelin, M.; Scaini, D.; Casani, R.; Aurand, E.R.; Padro, D.; Egimendia, A.; Cabrer, P.R.; Scarselli, M.; et al. Functional rewiring across spinal injuries via biomimetic nanofiber scaffolds. Proc. Natl. Acad. Sci. USA 2020, 117, 25212–25218.
- Sever-Bahcekapili, M.; Yilmaz, C.; Demirel, A.; Kilinc, M.C.; Dogan, I.; Caglar, Y.S.; Guler, M.O.; Tekinay, A.B. Neuroactive Peptide Nanofibers for Regeneration of Spinal Cord after Injury. Macromol. Biosci. 2021, 21, e2000234.
- Zhao, T.; Jing, Y.; Zhou, X.; Wang, J.; Huang, X.; Gao, L.; Zhu, Y.; Wang, L.; Gou, Z.; Liang, C.; et al. PHBV/PLA/Col-Based Nanofibrous Scaffolds Promote Recovery of Locomotor Function by Decreasing Reactive Astrogliosis in a Hemisection Spinal Cord Injury Rat Model. J. Biomed. Nanotechnol. 2018, 14, 1921–1933.
- Chedly, J.; Soares, S.; Montembault, A.; von Boxberg, Y.; Veron-Ravaille, M.; Mouffle, C.; Benassy, M.-N.; Taxi, J.; David, L.; Nothias, F. Physical chitosan microhydrogels as scaffolds for spinal cord injury restoration and axon regeneration. Biomaterials 2017, 138, 91–107.
- Cigognini, D.; Satta, A.; Colleoni, B.; Silva, D.; Donegà, M.; Antonini, S.; Gelain, F. Evaluation of Early and Late Effects into the Acute Spinal Cord Injury of an Injectable Functionalized Self-Assembling Scaffold. PLoS ONE 2011, 6, e19782.
- Palejwala, A.H.; Fridley, J.S.; Mata, J.A.; Samuel, E.L.G.; Luerssen, T.G.; Perlaky, L.; Kent, T.A.; Tour, J.M.; Jea, A. Biocompatibility of reduced graphene oxide nanoscaffolds following acute spinal cord injury in rats. Surg. Neurol. Int. 2016, 7, 75.
- Pawelec, K.M.; Koffler, J.; Shahriari, D.; Galvan, A.; Tuszynski, M.H.; Sakamoto, J. Microstructure and in vivo characterization of multi-channel nerve guidance scaffolds. Biomed. Mater. 2018, 13, 044104.
- Milbreta, U.; Nguyen, L.H.; Diao, H.; Lin, J.; Wu, W.; Sun, C.-Y.; Wang, J.; Chew, S.Y. Three-Dimensional Nanofiber Hybrid Scaffold Directs and Enhances Axonal Regeneration after Spinal Cord Injury. ACS Biomater. Sci. Eng. 2016, 2, 1319–1329.
- Tysseling, V.M.; Sahni, V.; Pashuck, E.T.; Birch, D.; Hebert, A.; Czeisler, C.; Stupp, S.I.; Kessler, J.A. Self-assembling peptide amphiphile promotes plasticity of serotonergic fibers following spinal cord injury. J. Neurosci. Res. 2010, 88, 3161–3170.
- Liu, Y.; Ye, H.; Satkunendrarajah, K.; Yao, G.S.; Bayon, Y.; Fehlings, M.G. A self-assembling peptide reduces glial scarring, attenuates post-traumatic inflammation and promotes neurological recovery following spinal cord injury. Acta Biomater. 2013, 9, 8075–8088.
- Wang, Y.; Tan, H.; Hui, X. Biomaterial Scaffolds in Regenerative Therapy of the Central Nervous System. BioMed Res. Int. 2018, 2018, 7848901.
- Furuya, T.; Hashimoto, M.; Koda, M.; Murata, A.; Okawa, A.; Dezawa, M.; Matsuse, D.; Tabata, Y.; Takahashi, K.; Yamazaki, M. Treatment with basic fibroblast growth factor-incorporated gelatin hydrogel does not exacerbate mechanical allodynia after spinal cord contusion injury in rats. J. Spinal Cord Med. 2013, 36, 134–139.
- Chvatal, S.A.; Kim, Y.-T.; Bratt-Leal, A.M.; Lee, H.; Bellamkonda, R.V. Spatial distribution and acute anti-inflammatory effects of Methylprednisolone after sustained local delivery to the contused spinal cord. Biomaterials 2008, 29, 1967–1975.
- Jain, A.; McKeon, R.J.; Brady-Kalnay, S.M.; Bellamkonda, R.V. Sustained Delivery of Activated Rho GTPases and BDNF Promotes Axon Growth in CSPG-Rich Regions Following Spinal Cord Injury. PLoS ONE 2011, 6, e16135.
- Wen, Y.; Yu, S.; Wu, Y.; Ju, R.; Wang, H.; Liu, Y.; Wang, Y.; Xu, Q. Spinal cord injury repair by implantation of structured hyaluronic acid scaffold with PLGA microspheres in the rat. Cell Tissue Res. 2016, 364, 17–28.
- Chen, B.; He, J.; Yang, H.; Zhang, Q.; Zhang, L.; Zhang, X.; Xie, E.; Liu, C.; Zhang, R.; Wang, Y.; et al. Repair of spinal cord injury by implantation of bFGF-incorporated HEMA-MOETACL hydrogel in rats. Sci. Rep. 2015, 5, 9017.
- Lin, J.; Anopas, D.; Milbreta, U.; Lin, P.H.; Chin, J.S.; Zhang, N.; Wee, S.K.; Tow, A.; Ang, W.T.; Chew, S.Y. Regenerative rehabilitation: Exploring the synergistic effects of rehabilitation and implantation of a bio-functional scaffold in enhancing nerve regeneration. Biomater. Sci. 2019, 7, 5150–5160.
- Shi, Q.; Gao, W.; Han, X.; Zhu, X.; Sun, J.; Xie, F.; Hou, X.; Yang, H.; Dai, J.; Chen, L. Collagen scaffolds modified with collagen-binding bFGF promotes the neural regeneration in a rat hemisected spinal cord injury model. Sci. China Life Sci. 2014, 57, 232–240.
- Wang, X.; Li, Y.; Gao, Y.; Chen, X.; Yao, J.; Lin, W.; Chen, Y.; Liu, J.; Yang, Y.; Wang, X. Combined use of spinal cord-mimicking partition type scaffold architecture and neurotrophin-3 for surgical repair of completely transected spinal cord in rats. J. Biomater. Sci. Polym. Ed. 2013, 24, 927–939.
- Li, G.; Che, M.-T.; Zhang, K.; Qin, L.-N.; Zhang, Y.-T.; Chen, R.-Q.; Rong, L.-M.; Liu, S.; Ding, Y.; Shen, H.-Y.; et al. Graft of the NT-3 persistent delivery gelatin sponge scaffold promotes axon regeneration, attenuates inflammation, and induces cell migration in rat and canine with spinal cord injury. Biomaterials 2016, 83, 233–248.
- Wei, Y.-T.; He, Y.; Xu, C.-L.; Wang, Y.; Liu, B.-F.; Wang, X.-M.; Sun, X.-D.; Cui, F.-Z.; Xu, Q.-Y. Hyaluronic acid hydrogel modified with nogo-66 receptor antibody and poly-L-lysine to promote axon regrowth after spinal cord injury. J. Biomed. Mater. Res. Part B Appl. Biomater. 2010, 95B, 110–117.
- Bighinati, A.; Focarete, M.L.; Gualandi, C.; Pannella, M.; Giuliani, A.; Beggiato, S.; Ferraro, L.; Lorenzini, L.; Giardino, L.; Calzà, L. Improved Functional Recovery in Rat Spinal Cord Injury Induced by a Drug Combination Administered with an Implantable Polymeric Delivery System. J. Neurotrauma 2020, 37, 1708–1719.
- Ehsanipour, A.; Sathialingam, M.; Rad, L.M.; de Rutte, J.; Bierman, R.D.; Liang, J.; Xiao, W.; Di Carlo, D.; Seidlits, S.K. Injectable, macroporous scaffolds for delivery of therapeutic genes to the injured spinal cord. APL Bioeng. 2021, 5, 016104.
- Xie, J.; Li, J.; Ma, J.; Li, M.; Wang, X.; Fu, X.; Ma, Y.; Yang, H.; Li, B. Saijilafu Magnesium Oxide/Poly(l-lactide-co-ε-caprolactone) Scaffolds Loaded with Neural Morphogens Promote Spinal Cord Repair through Targeting the Calcium Influx and Neuronal Differentiation of Neural Stem Cells. Adv. Healthc. Mater. 2022, 11, 2200386.
- Xi, K.; Gu, Y.; Tang, J.; Chen, H.; Xu, Y.; Wu, L.; Cai, F.; Deng, L.; Yang, H.; Shi, Q.; et al. Microenvironment-responsive immunoregulatory electrospun fibers for promoting nerve function recovery. Nat. Commun. 2020, 11, 4504, Erratum in Nat. Commun. 2021, 12, 2882.
- Rooney, G.E.; Knight, A.M.; Madigan, N.N.; Gross, L.; Chen, B.; Giraldo, C.V.; Seo, S.; Nesbitt, J.J.; Dadsetan, M.; Yaszemski, M.J.; et al. Sustained Delivery of Dibutyryl Cyclic Adenosine Monophosphate to the Transected Spinal Cord Via Oligo Hydrogels. Tissue Eng. Part A 2011, 17, 1287–1302.
- Stropkovska, A.; Kisucka, A.; Bimbova, K.; Bacova, M.; Galik, J.; Medvecky, L.; Sulla, I.; Karasova, M.; Lukacova, N. Combined therapy (Rho-A-kinase inhibitor and chitosan/collagen porous scaffold) provides a supportive environment for endogenous regenerative processes after spinal cord trauma. Arch. Ital. Biol. 2021, 159, 159–177.
- Smith, D.R.; Dumont, C.M.; Park, J.; Ciciriello, A.J.; Guo, A.; Tatineni, R.; Cummings, B.J.; Anderson, A.J.; Shea, L.D. Polycistronic Delivery of IL-10 and NT-3 Promotes Oligodendrocyte Myelination and Functional Recovery in a Mouse Spinal Cord Injury Model. Tissue Eng. Part A 2020, 26, 672–682.
- Breen, B.A.; Kraskiewicz, H.; Ronan, R.; Kshiragar, A.; Patar, A.; Sargeant, T.; Pandit, A.; McMahon, S.S. Therapeutic Effect of Neurotrophin-3 Treatment in an Injectable Collagen Scaffold Following Rat Spinal Cord Hemisection Injury. ACS Biomater. Sci. Eng. 2017, 3, 1287–1295.
- Jain, A.; Kim, Y.-T.; McKeon, R.J.; Bellamkonda, R.V. In situ gelling hydrogels for conformal repair of spinal cord defects, and local delivery of BDNF after spinal cord injury. Biomaterials 2006, 27, 497–504.
