Pore-based sensing is a highly sensitive sensing technology for the detection of extremely small particles such as molecules, proteins, and viruses (50–200 nm). Pore-based sensing is conducted by applying an electric field across nanopores, usually made of biomacromolecules, e.g., α-hemolysin or synthetic materials, e.g., graphene and semiconductor. When a particle passes through the pore, changes in the current waveform can be observed. The presence of specific waveform changes indicates the presence of target, and the number of this specific waveform can be used to determine the concentration. In this chapter, an overview of pore-based sensing technology is presented. Their applications in virus detection are discussed.
- virus detection
- pore-based assay
- nanopore
- particle detection
1. Introduction
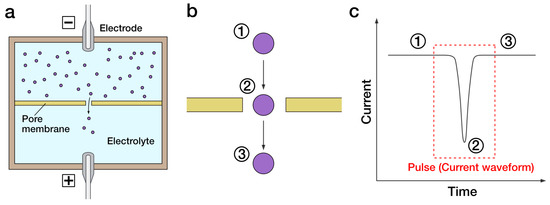
2. Fabrication of Pores for Sensing
3. Quantification of Virus Using Pore-Based Sensing
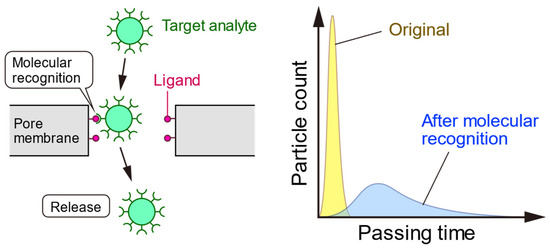
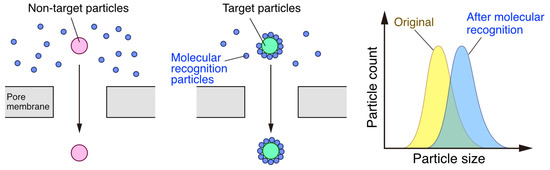
4. Advanced Techniques of Pore-Based Sensing for Virus Detection
This entry is adapted from the peer-reviewed paper 10.3390/s23156830
References
- Will Anderson; Darby Kozak; Victoria A. Coleman; Åsa K. Jämting; Matt Trau; A comparative study of submicron particle sizing platforms: Accuracy, precision and resolution analysis of polydisperse particle size distributions. J. Colloid Interface Sci. 2013, 405, 322-330, .
- Li-Qun Gu; Orit Braha; Sean Conlan; Stephen Cheley; Hagan Bayley; Stochastic sensing of organic analytes by a pore-forming protein containing a molecular adapter. Nat. 1999, 398, 686-690, .
- John J. Kasianowicz; Eric Brandin; Daniel Branton; David W. Deamer; Characterization of individual polynucleotide molecules using a membrane channel. null 1996, 93, 13770-13773, .
- Zuzanna Siwy; Pavel Apel; Dagmar Baur; Dobri D Dobrev; Yuri E Korchev; Reinhard Neumann; Reimar Spohr; Christina Trautmann; Kay-Obbe Voss; Preparation of synthetic nanopores with transport properties analogous to biological channels. Surf. Sci. 2003, 532-535, 1061-1066, .
- Jeffrey D. Uram; Kevin Ke; Alan J. Hunt; Michael Mayer; Label-Free Affinity Assays by Rapid Detection of Immune Complexes in Submicrometer Pores. Angew. Chem. Int. Ed. 2006, 45, 2281-2285, .
- C. Chad Harrell; Youngseon Choi; Lloyd P. Horne; Lane A. Baker; Zuzanna S. Siwy; Charles R. Martin; Resistive-Pulse DNA Detection with a Conical Nanopore Sensor. Langmuir 2006, 22, 10837-10843, .
- Shanshan Wu; Sang Ryul Park; Xinsheng Sean Ling; Lithography-Free Formation of Nanopores in Plastic Membranes Using Laser Heating. Nano Lett. 2006, 6, 2571-2576, .
- Jasper P. Fried; Yanfang Wu; Richard D. Tilley; J. Justin Gooding; Optical Nanopore Sensors for Quantitative Analysis. Nano Lett. 2022, 22, 869-880, .
- Jeffrey D. Uram; Kevin Ke; Alan J. Hunt; Michael Mayer; Submicrometer Pore-Based Characterization and Quantification of Antibody–Virus Interactions. Small 2006, 2, 967-972, .
- Van Etten, J.L. Algal Viruses (Phycodnaviridae). In Encyclopedia of Virology, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 1999; pp. 44–50.
- James L. Van Etten; Russel H. Meints; Giant Viruses Infecting Algae. Annu. Rev. Microbiol. 1999, 53, 447-494, .
- Z. S. Siwy; Ion-Current Rectification in Nanopores and Nanotubes with Broken Symmetry. Adv. Funct. Mater. 2006, 16, 735-746, .
- Tetsuya Yamaki; Nunung Nuryanthi; Hiroshi Koshikawa; Masaharu Asano; Shin-Ichi Sawada; Shin Hasegawa; Yasunari Maekawa; Kay-Obbe Voss; Christina Trautmann; Reinhard Neumann; et al. Investigation of Nanopore Evolution in Track-Etched Poly(vinylidene fluoride) Membranes. Trans. Mater. Res. Soc. Jpn. 2012, 37, 223-226, .
- Stephen J. Sowerby; Murray F. Broom; George B. Petersen; Dynamically resizable nanometre-scale apertures for molecular sensing. Sensors Actuators B: Chem. 2007, 123, 325-330, .
- Makusu Tsutsui; Sadato Hongo; Yuhui He; Masateru Taniguchi; Nobuhiro Gemma; Tomoji Kawai; Single-Nanoparticle Detection Using a Low-Aspect-Ratio Pore. ACS Nano 2012, 6, 3499-3505, .
- Sou Ryuzaki; Makusu Tsutsui; Yuhui He; Kazumichi Yokota; Akihide Arima; Takanori Morikawa; Masateru Taniguchi; Tomoji Kawai; Rapid structural analysis of nanomaterials in aqueous solutions. Nanotechnol. 2017, 28, 155501, .
- Gaurav Goyal; Rafael Mulero; Jamel Ali; Armin Darvish; Min Jun Kim; Low aspect ratio micropores for single-particle and single-cell analysis. Electrophor. 2015, 36, 1164-1171, .
- Akihide Arima; Makusu Tsutsui; Ilva Hanun Harlisa; Takeshi Yoshida; Masayoshi Tanaka; Kazumichi Yokota; Wataru Tonomura; Masateru Taniguchi; Mina Okochi; Takashi Washio; et al. Selective detections of single-viruses using solid-state nanopores. Sci. Rep. 2018, 8, 1-7, .
- G R Willmott; R Vogel; S S C Yu; L G Groenewegen; G S Roberts; D Kozak; W Anderson; M Trau; Use of tunable nanopore blockade rates to investigate colloidal dispersions. J. Physics: Condens. Matter 2010, 22, 454116, .
- Robert Vogel; Geoff Willmott; Darby Kozak; G. Seth Roberts; Will Anderson; Linda Groenewegen; Ben Glossop; Anne Barnett; Ali Turner; Matt Trau; et al. Quantitative Sizing of Nano/Microparticles with a Tunable Elastomeric Pore Sensor. Anal. Chem. 2011, 83, 3499-3506, .
- Darby Kozak; Will Anderson; Matthew Grevett; Matt Trau; Modeling Elastic Pore Sensors for Quantitative Single Particle Sizing. J. Phys. Chem. C 2012, 116, 8554-8561, .
- Fulya Akpinar; John Yin; Characterization of vesicular stomatitis virus populations by tunable resistive pulse sensing. J. Virol. Methods 2015, 218, 71-76, .
- Samuel Alizon; Christian Selinger; Mircea T Sofonea; Stéphanie Haim-Boukobza; Jean-Marc Giannoli; Laetitia Ninove; Sylvie Pillet; Vincent Thibault; Alexis de Rougemont; Camille Tumiotto; et al. Epidemiological and clinical insights from SARS-CoV-2 RT-PCR crossing threshold values, France, January to November 2020. Wkly. releases (1997–2007) 2022, 27, 2100406, .
- Daniel Evans; Simon Cowen; Martin Kammel; Denise M O’Sullivan; Graham Stewart; Hans-Peter Grunert; Jacob Moran-Gilad; Jasper Verwilt; Jiwon In; Jo Vandesompele; et al. The Dangers of Using Cq to Quantify Nucleic Acid in Biological Samples: A Lesson From COVID-19. Clin. Chem. 2021, 68, 153-162, .
- Yukichi Horiguchi; Yukio Nagasaki. Softinterface on Biosensing; Wiley: Hoboken, NJ, United States, 2016; pp. 211-219.
- Masaru Tanaka; Tomohiro Hayashi; Shigeaki Morita; The roles of water molecules at the biointerface of medical polymers. Polym. J. 2013, 45, 701-710, .
- R. Erik Holmlin; Xiaoxi Chen; Robert G. Chapman; Shuichi Takayama; George M. Whitesides; Zwitterionic SAMs that Resist Nonspecific Adsorption of Protein from Aqueous Buffer. Langmuir 2001, 17, 2841-2850, .
- Yukichi Horiguchi; Yuji Miyahara; Surface modification to suppress small pore clogging in resistive pulse sensing. Appl. Phys. Express 2020, 13, 115002, .
- Hongwen Wu; Yuhao Chen; Qizhao Zhou; Rongliang Wang; Baicheng Xia; Dejun Ma; Kaifu Luo; Quanjun Liu; Translocation of Rigid Rod-Shaped Virus through Various Solid-State Nanopores. Anal. Chem. 2016, 88, 2502-2510, .
- Yinghua Qiu; Preston Hinkle; Crystal Yang; Henriette E. Bakker; Matthew Schiel; Hong Wang; Dmitriy Melnikov; Maria Gracheva; Maria Eugenia Toimil-Molares; Arnout Imhof; et al. Pores with Longitudinal Irregularities Distinguish Objects by Shape. ACS Nano 2015, 9, 4390-4397, .
- Yulun Zhang; Martin A. Edwards; Sean R. German; Henry S. White; Multipass Resistive-Pulse Observations of the Rotational Tumbling of Individual Nanorods. J. Phys. Chem. C 2016, 120, 20781-20788, .
- R. Maugi; P. Hauer; J. Bowen; E. Ashman; E. Hunsicker; M. Platt; A methodology for characterising nanoparticle size and shape using nanopores. Nanoscale 2019, 12, 262-270, .
- Róbert E. Gyurcsányi; Chemically-modified nanopores for sensing. TrAC Trends Anal. Chem. 2008, 27, 627-639, .
- Ruoshan Wei; Volker Gatterdam; Ralph Wieneke; Robert Tampé; Ulrich Rant; Stochastic sensing of proteins with receptor-modified solid-state nanopores. Nat. Nanotechnol. 2012, 7, 257-263, .
- Ren Ren; Yanjun Zhang; Binoy Paulose Nadappuram; Bernice Akpinar; David Klenerman; Aleksandar P. Ivanov; Joshua B. Edel; Yuri Korchev; Nanopore extended field-effect transistor for selective single-molecule biosensing. Nat. Commun. 2017, 8, 1-9, .
- Akihide Arima; Ilva Hanun Harlisa; Takeshi Yoshida; Makusu Tsutsui; Masayoshi Tanaka; Kazumichi Yokota; Wataru Tonomura; Jiro Yasuda; Masateru Taniguchi; Takashi Washio; et al. Identifying Single Viruses Using Biorecognition Solid-State Nanopores. J. Am. Chem. Soc. 2018, 140, 16834-16841, .
- T. Miyagawa; S. Hongo; N. Nakamura; Y. Horiguchi; Y. Miyahara; H. Shibata. A Novel Diagnostic System for Infectious Diseases Using Solid-State Nanopore Devices; Institute of Electrical and Electronics Engineers (IEEE): Piscataway, NJ, United States, 2018; pp. 2833-2836.
- Yukichi Horiguchi; Norihiko Naono; Osamu Sakamoto; Hiroaki Takeuchi; Shoji Yamaoka; Yuji Miyahara; Methodology to Detect Biological Particles Using a Biosensing Surface Integrated in Resistive Pulse Sensing. ACS Appl. Mater. Interfaces 2022, 14, 20168-20178, .
- Defang Ding; Pengcheng Gao; Qun Ma; Dagui Wang; Fan Xia; Biomolecule‐Functionalized Solid‐State Ion Nanochannels/Nanopores: Features and Techniques. Small 2019, 15, e1804878, .
- Yukichi Horiguchi; Tatsuro Goda; Akira Matsumoto; Hiroaki Takeuchi; Shoji Yamaoka; Yuji Miyahara; Gold Nanoparticles with Ligand/Zwitterion Hybrid Layer for Individual Counting of Influenza A H1N1 Subtype Using Resistive Pulse Sensing. Langmuir 2018, 35, 1798-1806, .
- Akihide Arima; Makusu Tsutsui; Takeshi Yoshida; Kenji Tatematsu; Tomoko Yamazaki; Kazumichi Yokota; Shun’ichi Kuroda; Takashi Washio; Yoshinobu Baba; Tomoji Kawai; et al. Digital Pathology Platform for Respiratory Tract Infection Diagnosis via Multiplex Single-Particle Detections. ACS Sensors 2020, 5, 3398-3403, .
- Masateru Taniguchi; Shohei Minami; Chikako Ono; Rina Hamajima; Ayumi Morimura; Shigeto Hamaguchi; Yukihiro Akeda; Yuta Kanai; Takeshi Kobayashi; Wataru Kamitani; et al. Combining machine learning and nanopore construction creates an artificial intelligence nanopore for coronavirus detection. Nat. Commun. 2021, 12, 1-8, .
