Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Pharmacology & Pharmacy
The number of patients with ocular disorders has increased due to contributing factors such as aging populations, environmental changes, smoking, genetic abnormalities, etc. Age-related macular degeneration (AMD) is one of the common ocular disorders which may advance to loss of vision in severe cases. The advanced form of AMD is classified into two types, dry (non-exudative) and wet (exudative) AMD.
- age-related macular degeneration
- drug delivery
- intravitreal route
1. Introduction
Age-related macular degeneration (AMD) is one of the common causes of irreversible loss of vision in individuals above 65 years [1,2]. According to the World Health Organization (WHO), the number of AMD cases at present has increased steadily to 196 million, which is expected to accumulate to about 288 million by 2040 [3,4]. It contributes to about 10% of blindness throughout the world by hampering the regular functions of photoreceptors, the retinal pigment epithelium (RPE), and the choroid [5]. Age is the main contributing factor, as people over 85 years of age are ten times more susceptible to AMD [6]. Along with this, genetic background, environmental factors, smoking, para-inflammation, etc. are other important factors [6,7]. However the adoption of a healthy lifestyle for most of the patients with AMD could be useful, which is even more beneficial for patients with a high genetic predisposition. The risk of progression to late-stage AMD can be reduced by half by incorporating an intake of vegetables, fruits, fish, etc. in the diet [8,9]. Quitting smoking is another lifestyle change to combat AMD because active smokers are susceptible to AMD at a rate two to three times that of non-smokers [10]. The risk of genetic factors in AMD is more important in younger individuals. Examples would be variations in genes involved with the complement system and histocompatibility locus antigen (HLA) genes [11,12]. The contribution of other factors such as cardiovascular conditions, exposure to sunlight, alcohol consumption, etc. in AMD progression is unclear [13].
Based on pathophysiologic features, AMD is majorly classified into dry and wet-type. The initial presentation of the disease is the dry or non-exudative type, which may further advance in the later stage into wet or neovascular AMD [14,15]. Although dry AMD accounts for about 90% of overall cases and wet AMD is responsible for only about 10% of cases, severe loss of vision is associated with the latter form [16]. The clinical feature of the disease involves distinct drusen accumulations and pigment vicissitudes in the early stages, leading to geographic atrophy and neovascularization in the later stages of AMD [17]. Drusen are characteristic epithelial deposits composed of lipids, proteins, etc. that are formed as tiny deposits in yellow color [18]. Typically, drusen are found between the basal membrane of RPE and the inner collagenous region of Bruch’s membrane (Figure 1). The occurrence of drusen in the macula marks the incidence of age-related macular degeneration; the size and area of the drusen may also indicate the progression of AMD in the advanced stage [19,20]. The types of drusen in the macula of AMD patients include the hard and discrete type or the soft and diffusive type. The soft and diffusive drusen are considered to be more pathogenic and tend towards choroidal neovascularization (CNV) [21]. Geographic atrophy (GA) is merged regions formed due to dead RPE cells which are covered by the atrophic photoreceptor. It appears initially in the parafoveal region and may later progress into the foveal regions [22]. CNV involves the formation of newer blood vessels around the RPE, or may infiltrate subretinal space. It mostly contributes to impaired and leaking vessels, followed by the accumulation of blood and fluid in the macula [20,23].
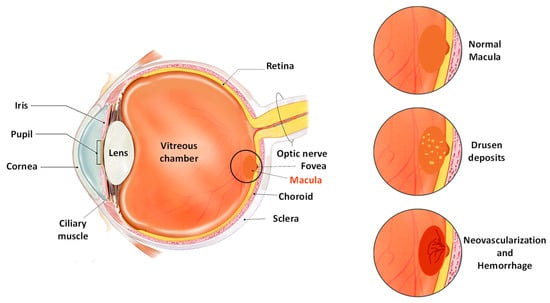
Figure 1. Pictorial representation of the anatomy of the eye in individuals with AMD.
The pathologic mechanisms involved in the development and progression of AMD are mainly related to the impairment and degeneration of RPE [24]. The rise in oxidative load and dysfunction of defensive antioxidant mechanisms has also been recognized as one of the key risk factors affecting the progression of the disease. The oxidative degradation with the advancement of age results in the anatomical degeneration of choriocapillaris, subsequently contributing to the reduction in supply of blood to the RPE and photoreceptors [5,25]. The impeded circulation decreases the usual elimination of lipids, proteins, and other byproducts, which accumulate in the form of drusen [18,21]. These accumulations induce the reformation of the extracellular matrix and incite an inflammatory reaction. Owing to the intertwining of such intricate pathologic processes, AMD progresses to atrophy or neovascularization [26,27].
2. Novel Drug Delivery Systems for AMD
The delivery systems used for therapeutic agents against AMD primarily revolve around using either implantable devices or intravitreal injectable liquids. Recently, scientists have made considerable attempts at improving the potential of therapeutic agents used for AMD by designing novel carriers that can reduce invasiveness at a low cost [31,79]. Despite technological developments achieved for the delivery of ophthalmic agents, there is still a need for a more reliable approach owing to the bioavailability and penetrability issues presented due to ocular barriers. In the case of reduced bioavailability, the viability of therapeutic agents for AMD becomes hampered, as they require frequent administration [80]. The approaches under investigation have attempted to improve the bioavailability of the drugs either by using nanoparticulate carriers [81], sustained-release products [82], or penetration enhancers [83]. The novel drug delivery systems targeting different routes of administration (Figure 2), with a special emphasis on their applications and limitations.
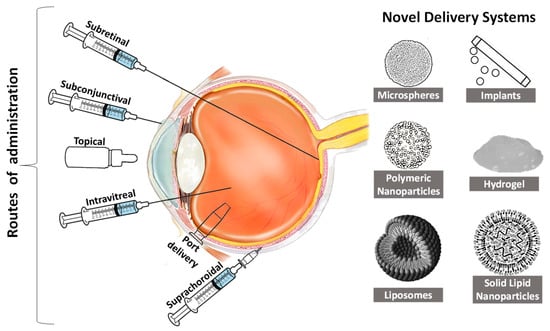
Figure 2. Routes of administration for the ocular drug delivery system for age-related macular degeneration.
2.1. Intravitreal Route of Delivery
Intravitreal space is the most employed route for the delivery of therapeutic agents for AMD. The main reason behind the popularity of this route is its high safety, minimum invasiveness, convenient application, and adequate efficacy [84]. Macular edema is one of the common features of neovascular or wet AMD, and intravitreal antibodies targeting VEGF have been shown to decrease macular edema to further avoid loss of vision. Therefore, all of the therapies that are intravitreally injected target wet AMD [85]. The microspheres containing poly(D, L-lactide-co-glycolide)/poly(cyclohexane-1,4-diyl acetone dimethylene ketal) (PLGA/PCADK) were produced using a solid-in-oil-in-water emulsification method. The smooth spherical microspheres showed an initial burst release, followed by a sustained release for about 50 days. Adequate encapsulation efficiency and better tolerability to ocular tissues make these composite microspheres a potential candidate for delivering drugs against ocular diseases [86]. PLGA-based nanoparticles were fabricated to enhance the shelf-life and stability of bevacizumab, along with imparting a controlled release feature. The negative zeta potential (−23.1 mV) and high encapsulation efficiency (82.47%) indicated the adequate stability and efficiency of the nanoparticle formulation. The pH-dependent release of bevacizumab was also observed over 168 h, and the release of the drug was significantly higher at pH 10 than at pH 6 and 7.4. MTT and bromodeoxyuridine (BrdU) proliferation assays showed no significant differences in the bioactivity of bevacizumab when loaded into PLGA nanoparticles [87]. Another attempt of developing PLGA nanoparticles of bevacizumab was carried out to increase its residence time in the aqueous and vitreous humor for prolonging the duration of action. The study reported that nanoparticles demonstrated the enhancement of bioavailability and the anti-VEGF activity of bevacizumab, along with no major signs of cytotoxicity and tissue toxicity. The incorporation of PLGA further improved the anti-angiogenic effect by suppressing corneal and retinal neovascularization [88].
The mesoporous silica nanoparticles (MSNs) functionalized with 3-aminopropyltriethoxysilane (3-aminopropyl) triethoxysilane (APTES) and mPEG-succinimidyl carboxymethyl ester (mPEG-NHS) were also reported to show improvement in the anti-VEGF potential of bevacizumab. In vitro studies showed that MSNs were effective in inhibiting proliferation, migration, and tube formation of endothelial cells induced by VEGF. Furthermore, the MSNs exhibited in vivo inhibition of corneal and retinal neovascularization [89]. Bevacizumab containing chitosan nanoparticles was developed using the ionic gelation method meant for embedding in the ocular implant. The homogeneously formed nanoparticles (particle size ~78.5 nm) displayed the extended release of bevacizumab over a 2-month study [90]. The preparation of a chitosan grafted-poly(ethylene glycol) methacrylate-(CS-g-PEGMA) based polymeric nanocarrier of bevacizumab was carried out by the first synthesis of CS-g-PEGMA by the Michael addition reaction, and nanoparticles were designed by the double crosslinking of reverse emulsion. The study reported uniform spherical particles that possess pH-sensitive properties in aqueous conditions. The nanoparticles showed the controlled release of bevacizumab for more than 72 h due to the swelling tendency in aqueous solution. The preparation designed for local injection was found to be completely hemocompatible without any significant toxicity indications [91]. Mu et al. prepared multivesicular liposomes of bevacizumab by the double emulsification technique and the use of 10% human serum albumin preserved the activity of bevacizumab for a longer duration. The bevacizumab was released from the liposomes in a sustained fashion for up to 14 days, owing to slow erosion and diffusion from the vesicles. The lesions of CNV were reduced in the rats after 28 days of treatment with bevacizumab containing liposomes due to the prolonged retention in the vitreous humor [92].
Vollrath et al. developed sustained-release solid lipid implants of ranibizumab using the twin-screw extrusion method. The implant could load a high amount of protein (3 mg/implant) with consistently sustained release profiles for 120 days. The implant showed a predominantly high release of the monomeric form (>95%) of the ranibizumab initially, followed by the formation of the hydrophobic type upon the completion of 18 weeks. The stability of the ranibizumab in the implant was exceptional with no signs of aggregation or alterations in secondary structures [93]. PLGA microparticles have been investigated for fabricating sustainable release systems of mABs such as ranibizumab for enhancing their anti-angiogenic potential and protection against proteolytic degradation. The microparticles sustained the release of ranibizumab, and about 80% release was achieved after the completion of 3 weeks. The cell proliferation and tube formation assay of the formulation showed a considerable reduction in VEGF-induced tube formation [94]. PLGA-based microspheres of ranibizumab showed a significantly greater reduction in lesions of CNV in studied animals at lower doses. Apart from the mild and insignificant rise in intraocular pressure, there was no signal of cellular dysfunction of the retina in the electroretinogram [95]. The biodegradable microsphere-based hydrogel of ranibizumab was designed by Liu et al. to impart controlled release characteristics. Volume phase transition temperature (VPTT) showed an increase while swelling ratios decreased with the corresponding increment in the concentration of a cross-linker. The microspheres demonstrated the pH-sensitive controlled release of ranibizumab for up to 6 months, and relatively rapid release can be attained by increasing the concentration of the degradable cross-linking agent [96].
The researchers used a similar delivery system for the encapsulation of aflibercept. The rate and extent of release of aflibercept was also in a controlled manner depending upon the concentration of the cross-linking agent and the loaded microspheres. The drug released from the microspheres showed no signs of cytotoxicity from its degraded byproducts, and bioactivity was maintained throughout the complete release period [97]. The prepared formulation was injected into the rhesus macaques and observed in the vitreous fluid for 6 months after injection. There were no signs of alterations in the anatomy and physiology of the retina, along with the observation of about 2.1 ng/µL of aflibercept in the vitreous [98]. Polymeric nanoparticles of aflibercept also depicted the sustained release of the protein over 7 days. The nanoparticles showed uniform distribution with insignificant signs of toxicity in the ARPE-19 cells [99]. Adamson et al. reported the production and characterization of microparticles of PolyActive™ hydrogel co-polymer. The outcomes of the study demonstrated the sustained delivery of domain antibodies from microparticles in the rabbit and primate eyes for 6 months. A sustained release intravitreal implant of dexamethasone was prepared, and drug release behavior was studied using various dissolution conditions and methods, such as the shaking incubator experiment, the EyeMovement System (EyeMoS), the USP apparatus 7, and the Vitreous Model. The outcomes of the drug release from different test media, apparatus, and methods displayed high variation. Furthermore, the models and conditions were only able to depict the release of the drug from a gelled compartment, and none of the techniques were able to adequately predict the in vivo performance of the implants [101].
2.2. Delivery through Subretinal Space
The region between the RPE layer and the photoreceptors is considered as a subretinal space which allows the direct delivery of drugs to the RPE and photoreceptor cells. The subretinal injections utilize trans-scleral and trans-corneal routes in animal studies for attaining the required desired concentration of a drug in subretinal space [113,114]. It involves the prior conducting of a vitrectomy to separate the posterior vitreous that may use acetonide triamcinolone for better visualization [114]. The subretinal injection primarily involves three approaches/routes for the administration of drugs: (a) the transcorneal route crossing through the pupil, lens, vitreous region, and retina; (b) the transscleral route through the limbus region and passing through the vitreous; and (c) the transscleral route crossing through the Bruch’s membrane and choroid [114]. Figure 3 illustrates the approaches for subretinal injection adopted by various researchers for the delivery of drugs for AMD. However, the subretinal delivery is associated with challenges due to high invasiveness and access to a small area upon every injection. Despite these obstacles, researchers have become interested in targeting the subretinal space for gene delivery and cell therapy [115]. Palucorcel is a newly developed cell-based therapy for AMD which contains human umbilical tissue-derived cells in a cryopreserved product [116]. A novel subretinal delivery injection of palucorel was evaluated for safety and efficacy in patients with GA. The treated patients showed mild and non-critical adverse events with no signs of retinal detachment or alterations in intraocular pressure. However, the palucorel was successfully delivered through the subretinal site, but the reduction in GA and improved visual acuity were not exhibited throughout the study [117].
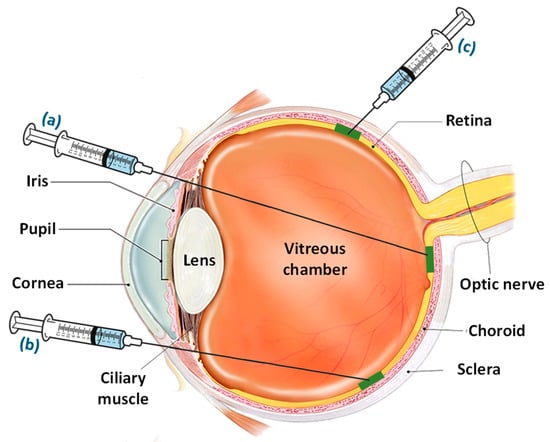
Figure 3. Approaches/routes for subretinal injection including (a) the transcorneal route entering through the pupil and passing through the lens, vitreous region, and retina, and (b) the transscleral route passing through the limbus region and vitreous, opposite to the retinal region into subretinal space, and (c) the transscleral route crossing through the Bruch’s membrane and choroid.
Gene therapy is one of the effective approaches for dealing with AMD by incorporating healthy genes in the cells of patients to avoid or treat defective genetic pathways. The benefit of using gene therapy is that it provides long-lasting treatment and enables targeted ocular regions to generate their protective agents [118]. Streptococcus pyogenes Cas9 (SpCas9) mRNA targets VEGFa and Rho genes in RPE and photoreceptor cells by using SpCas9 or small nucleases in adeno-associated viruses. They are employed in the treatment of Leber congenital amaurosis type 10, which occurs due to mutations in the CEP290 gene [119]. A subretinal injection comprising SpCas9 mRNA and expression cassettes were found to be effective in wet-AMD. The modified lentiviruses inhibited VEGFa in RPE, resulting in a 63% reduction in choroidal neovascularization without affecting undesirable target edits and immune responses. This approach may also be suitable for other forms of retinal disorders wherein the restriction of neovascularization is required [120]. A lentiviral gene therapy vector (RetinoStat®) based on an equine infection-causing anemia virus was developed for delivering two anti-angiogenic genes (endostatin and angiostatin) to the retina to suppress angiogenesis and enhance the vision of patients [121]. RetinoStat® was studied for subretinal delivery for the management of wet-AMD. The amount of endostatin and angiostatin increased after subretinal administration in rabbit eyes throughout the study. Ocular inflammation was reduced with 1 month of continuous dosing, with no considerable changes in electroretinograms and intraocular pressure [122]. The recombinant adeno-associated virus- (rAAV) based gene-therapy allows vector-like, soluble fms-like tyrosine kinase-1 (sFLT-1) to be delivered directly to the RPE and photoreceptor cells. This enables the uptake and transduction of viral vectors and sFLT-1 to express through protein-generating mechanisms of the cells [123]. A phase I trial of the subretinal injection of rAAV sFLT-1 demonstrated no proliferation in RPE cells, retinal scar production, or atrophic changes. However, some of the individuals encountered hemorrhages and cataract development. Overall, the product was well-tolerated and suitable for prolonged treatment for wet AMD [124]. Lambert et al. reported the investigation involving subretinal injections of adeno-associated virus-mediated gene therapy for targeting subretinal and outer retinal tissues with the cartilage oligomeric matrix protein angiopoietin-1 in mice simulated laser-assisted wet AMD. The results showed a reduction of about 29 to 33% in VEGF levels and 60 to 70% in the volume of choroid neovascularization. The vector-based product is appropriate for subretinal delivery and may serve as a promising treatment for neovascular AMD along with anti-VEGF agents [125].
2.3. Delivery through Suprachoroidal Space
The novel drug delivery techniques have facilitated better access to the suprachoroidal space for the treatment of ocular diseases. The drugs administered through the suprachoroidal space allow the attainment of higher concentrations in the retinal region, thereby reducing the undesirable delivery to the anterior ocular areas [126]. A novel antineoplastic agent called axitinib has a potent blocking activity over VEGF and platelet-derived growth factor (PDGF) receptors, therefore axitinib helps in the neovascularization and treatment of AMD [127]. The injectable suspension of axitinib (CLS-AX) was designed as a long-acting preparation for neovascular AMD. The ocular distribution demonstrated higher localization of axitinib in the sclera, RPE, and choroid, followed by the retina and vitreous. The product showed a marked reduction in the eye lesions in the rats, good tolerability, and no signs of toxicity [128]. Hancock et al. studied the bioavailability and sustainability of the small molecule suspension of A01017 (complement factor D inhibitor). The suprachoroidal injection was tolerated adequately in rabbits with minimum signs of toxicity. The suspension showed high sustained exposure of A01017 to the RPE, choroid, and sclera, along with first-order elimination throughout the 92-day study period [129].
The suprachoroidal graft of autologous cells was also proposed as a treatment for dry AMD, owing to its impact on the enhancement of visual acuity and microperimetric responses. The technique involved the implantation of adipose stem cells in the suprachoroidal space to stimulate the secretion of growth factors. The outcome was a significant improvement in visual acuity after six months, along with the maintenance of growth factor secretion and choroidal flow [130]. The restoration effect of grafted autologous cells on retinal cells was investigated to assess the continuous secretion of growth factors in patients with dry AMD. The best corrected visual acuity was found to be significantly improved in patients with higher retinal thickness averages due to the availability of greater cellularity [131].
Implantation containing adipose tissue-derived mesenchymal stem cells was evaluated for its safety and efficiency in patients with dry AMD. There were no occurrences of systemic or ocular complications in any of the patients with improvement in the visual field, visual acuity, and mf-ERG recordings [132]. Zhang et al. assessed the intraocular cell technology-based implant containing ciliary neurotrophic factor for the management of GA. The thickness of the retina increased with the incorporation of treatment in a dose-dependent manner, subsequently stabilizing the visual acuity. The implant delivered with newer technology was adequately tolerable by the patients and retarded the sequences of vision loss in GA [133]. The biodegradable nanoparticles can be used for the delivery of the VEGF-binding protein expression plasmid to RPE or even the entire eye for a longer duration through suprachoroidal injection. The anti-VEGF activity of nanoparticles was indicated through the repression of vascular leakage and neovascularization. The therapeutic benefits were further displayed by a considerable rise in sFlt1 retinal protein upon 1 month of therapy [134]. Patel et al. reported that the suprachoroidal delivery of aflibercept showed a reduction of the neovascular area in laser-induced neovascularized rat models. The treated animals showed a marked reduced neovascular leak area from 4862 pixels2 to 3318 pixels2 during the 21-day study period. The researchers suggest that suprachoroidal injection showed promise for the delivery of other anti-VEGF agents, especially in cases of wet AMD [135].
2.4. Port Delivery System
A port delivery system (PDS) encompasses a robust reservoir fabricated for the prolonged delivery of the drug into the vitreous cavity after being implanted. The ability of PDS to release the anti-VEGF medication for a longer duration helps in reducing the overdependency on intravitreal injections for the treatment of AMD. PDS is applied through the surgical insertion of the device into the scleral space through conjunctival peritomy. After implantation, the drug diffuses from the release control element in a sustained manner into the vitreous, which can be filled again once emptied [136]. A phase 2 trial of PDS of ranibizumab (10 to 100 mg/mL) was conducted for assessing its safety and efficacy. PDS implant insertion and refilling procedures were endured by the patients with a reduction in postoperative vitreous hemorrhage rate to 4.5% and no signs of implant clogging. Furthermore, the vision and structural outcomes after 9 months of administration of PDS (100 mg/mL) were similar to that of intravitreal ranibizumab (0.5-mg injection) [76]. Wykoff et al. discussed the pharmacokinetic outcomes of PDS of ranibizumab measured in the samples collected from serum and aqueous humor of the patients. The median serum concentrations of ranibizumab for PDS 10 mg/mL were found to be lower than the serum concentration achieved from intravitreal ranibizumab (0.5-mg injection). On the other hand, the median serum concentration resulting from 40 mg/mL and 100 mg/mL PDS were within the range of monthly intravitreal 0.5-mg injection throughout the 12-month study period [137]. Apart from this, the PDS of ranibizumab is also commercially viable owing to the sustained delivery of the drug, which reduces the dosing frequency. This results in lowering the cost of treatment, thereby reducing the burden of the treatment to both the patient and the health care system [138]. A phase 3 study of 24-week dosing with PDS ranibizumab is undergoing for evaluating its safety and efficacy in comparison with monthly intravitreal injections of ranibizumab. The constant ranibizumab delivery with PDS (refill after 6 months) demonstrated efficacy comparable to the intravitreal injections with more than 98% of the patient not requiring any supplemental treatment as observed for the first 6 months [139].
2.5. Delivery through Other Routes
Several studies have explored the utilization of some of the less preferred routes of administration to combat the limitations associated with existing therapies. Some of the routes reported in recent times chosen for drug delivery for AMD are the subconjunctival, topical, oral, etc. The subconjunctival region is located under the conjunctival membrane covering the sclera. The subconjunctival space is mostly chosen for delivering drugs to anterior ocular regions [140]. A depot formulation of sirolimus (mTOR inhibitor) was designed to be administered as a subconjunctival injection for the treatment of GA. The drug in its carrier formulation is well-tolerated in patients without any significant indications of adverse reactions. However, the outcomes of the study were not favorable concerning the structural and functional impact of therapy. There was an evident increment in GA areas and receding visual acuity in individuals after 24 months. Furthermore, there were no significant differences in the retinal thickness, drusen area, and macular sensitivity over 24 months [141]. Chaw et al. developed liposomal nanocarriers meant for subconjunctival administration and assessed their in vivo biodistribution using fiberoptic Confocal Laser Microendoscopy and radiotracing. Large positively charged liposomes demonstrated retention around the injection site for a longer duration, while neutral/negative small-sized liposomes showed better distribution in the limbus region. The nanocarriers can be optimized for encapsulation and controlled delivery of the drugs and biologicals used in AMD treatment [142].
Numerous efforts were also made to deliver drugs and biologicals through the topical route owing to convenient and non-invasive administration [143]. A topical product (PAN-90806) which inhibits the tyrosine kinase inhibitor of VEGF-A and Platelet-derived growth factor (PDGF) was subjected to a Phase I/II trial. According to the results, about 50% of the patients under treatment did not need any rescue therapy, and more than 80% showed improvement [144]. Danis et al. reported the development of another VEGF-A and PDGF inhibitor topical formulation (Pazopanib). A randomized trial has been conducted for examining their safety and efficacy in the treatment of wet AMD. The outcomes showed that there was no considerable reduction in retinal thickness, except for those having the CFH-TT genotype and receiving treatment three times daily (5 mg/mL) [145]. The Pazopanib eye drops in combination with ranibizumab did not show any therapeutic superiority over existing ranibizumab therapy. Consequently, the development of the product has been stopped [146]. Cogan et al. designed topical formulations of ranibizumab and bevacizumab delivered using cell-penetrating peptides (CPPs). The in vitro studies showed no toxicity from CPPs. The clinically requisite concentrations of CPPs along with anti-VEGF agents were detected in the posterior region of the rat eye. The efficiency of a daily administered topical agent in decreasing the choroidal neovascularization was comparable to the intravitreal injection of anti-VEGF agents [147].
Although the oral delivery of drugs provides a means of convenient and non-invasive administration, it is rarely chosen for the treatment of AMD due to the presence of ocular barriers, preventing effective delivery to the posterior regions. The phase II trial of an oral tablet formulation (X-82) containing anti-VEGF/PDGF agents was conducted to examine the efficacy of wet AMD. Although the product showed comparable improvement in visual acuity at higher doses, the limitations in safety and tolerability of the formulation resulted from the halt in further development. A case-control study reported the correlation between the oral administration of metformin and a reduction in the chances of developing AMD. The assessments indicate that metformin may possess therapeutic potential for AMD. However, there is a need for a comprehensive clinical study to assert the benefits of oral metformin therapy in preventing AMD development [148]. Stewart et al. reported a multicenter Phase 2a pilot clinical trial conducted over an orally administered product (AKST4290) for AMD. AKST4290 inhibits C-C Motif Chemokine Receptor 3 (CCR3), an eotaxin receptor that suppresses inflammation and neovascularization for the management of wet AMD. The study showed a substantial enhancement in the visual acuity of the patients with no indications of severe adverse events. It was suggested that further studies will be carried out as randomized controlled trials with a placebo in order to be assured about the safety of the product [149].
This entry is adapted from the peer-reviewed paper 10.3390/life13020568
This entry is offline, you can click here to edit this entry!
